Figure 13.
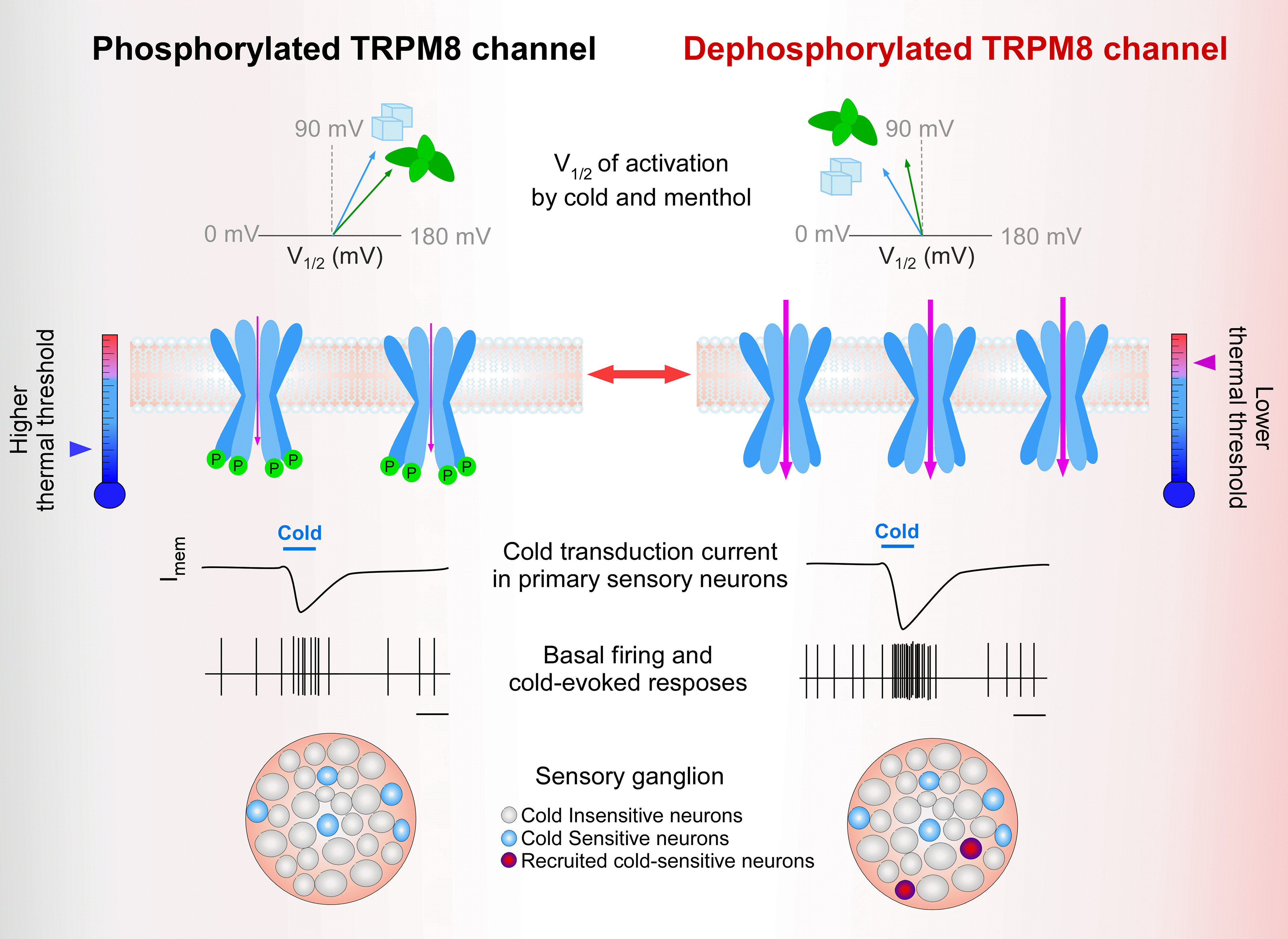
Graphical representation of the effect of basal phosphorylation on TRPM8 channel function. Left column, Schematic representation of V1/2 (top row), and membrane availability (second row) and functional properties (lower rows) of phosphorylated TRPM8 channels under basal conditions. Arrow at the thermometer indicates the mean thermal threshold of the cell as the results of this set of parameters. Right column, Potentiated responses to cold and menthol of unphosphorylated TRPM8 channels are because of a shift in the activation V1/2 toward more negative membrane potentials and an increase in the number of active channels at the plasma membrane. The number of channels in the scheme at right reflects the expected changes in expression level at the plasma membrane of dephosphorylated TRPM8 channels. Basal kinase activity downregulates TRPM8 function in cold thermoreceptor neurons, suggesting that cold sensing could be fine-tuned by controlling the phosphorylation state of the channel and adjusting the population of CSNs (third to fifth row). Among the basally phosphorylatable amino acids we identified, serine 29 is the most relevant phospho-residue that negatively modulates TRPM8 in resting conditions, acting as a reversible brake on channel function.
