Fig. 1. Design and characterization of Voltair probes:
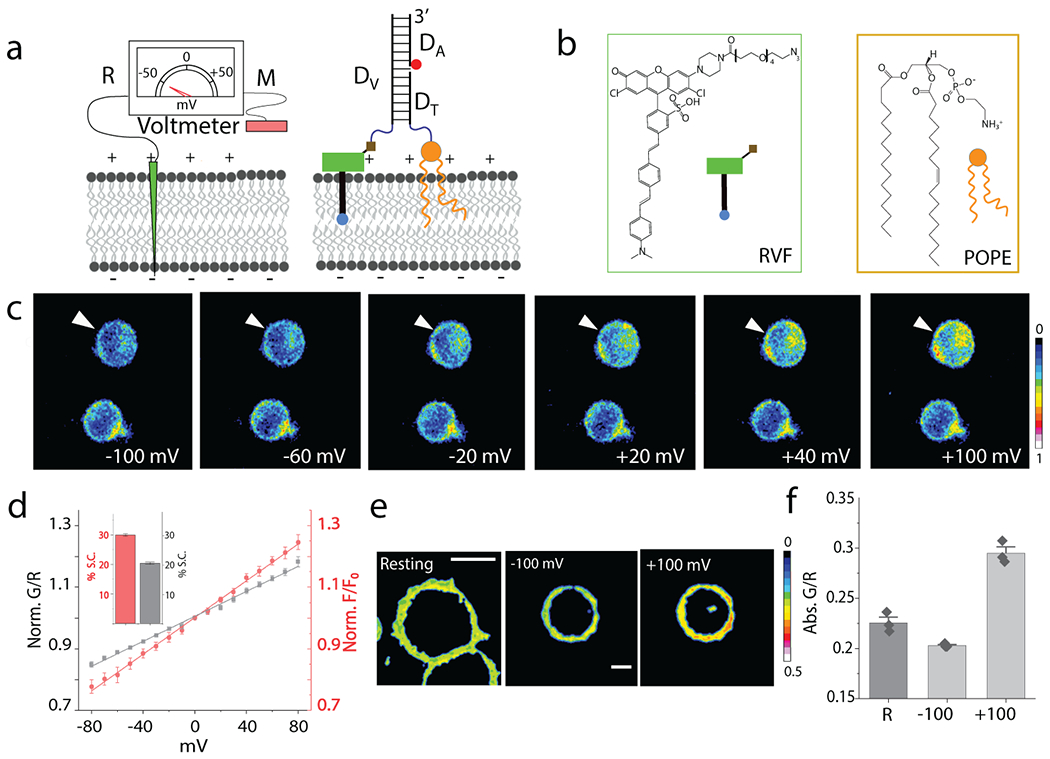
(a) Schematic of the working principle of DNA voltmeter Voltair: Measuring probe (M, Green) is a voltage sensitive dye (RVF) conjugated to a DNA duplex that is membrane-tethered by attachment to a lipid anchor (POPE). Reference probe (R, red) is DNA duplex with a reference dye (Atto647N, red sphere) that together with RVF reports membrane potential ratiometrically. (b) Structure of a conjugatable version of RVF (RVF-N3) and the lipid anchor POPE. (c) Pseudo-color images show pixel-wise ratio of RVF to Atto647N fluorescence intensities (G/R) as a function of membrane potential applied by whole cell voltage clamping. Voltage clamped cell shown by white arrow head. Scale: 10 μm. (d) Sensor response of VoltairPM (gray trace) and unmodified RVF (red trace) as a function of plasma membrane potential in HEK 293T cells. G/R values are normalized to that at 0 mV. Inset: Percentage signal change (S.C.) of VoltairPM (black) and unmodified RVF (red) from 0 to 100 mV. Error bar represents mean ± s.d. from n = 6 cells. Experiments were repeated thrice independently with similar results. (e, f) Absolute G/R values of the plasma membranes of resting cells (Res) and cells voltage clamped at −100 mV and +100 mV. Error bar represents mean ± s.e.m. of three independent trials, n = 15 cells. Scale: 10 μm.
