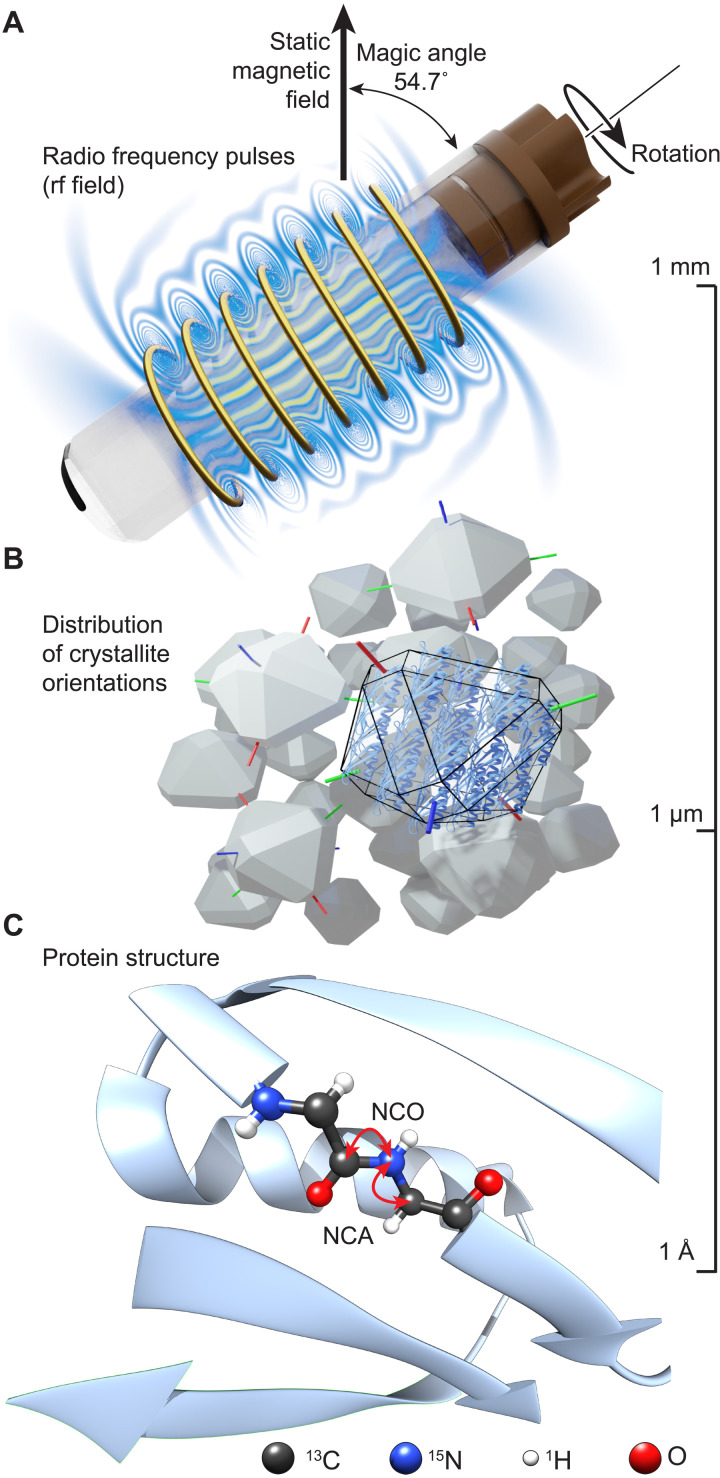
Fig. 1. Experimental setup of the MAS solid-state NMR experiment of insoluble proteins.
(A) The sample is placed inside a rotor that is oriented at 54.7° with respect to the static magnetic field and rotated within a solenoid coil, which allows the application of rf pulses to manipulate nuclear magnetization. Upon sample rotation, molecules experience periodical modulations of the rf field due to spatial inhomogeneity. Magnetic field lines are drawn schematically. (B) Protein molecules are contained in microcrystals that are randomly oriented in a powder. (C) Atomic level protein structure with arrows illustrating NCA and NCO magnetization transfer pathways between the amide nitrogen and Cα/C′ carbons (NCA/NCO transfers) of the protein backbone. The relative orientation of a bond vector with respect to the external static magnetic field determines the size of the dipolar interaction between the two atoms. The scale on the right indicates typical order of magnitude for object dimensions.