Fig. 1 |. Comparison of TCR docking modes.
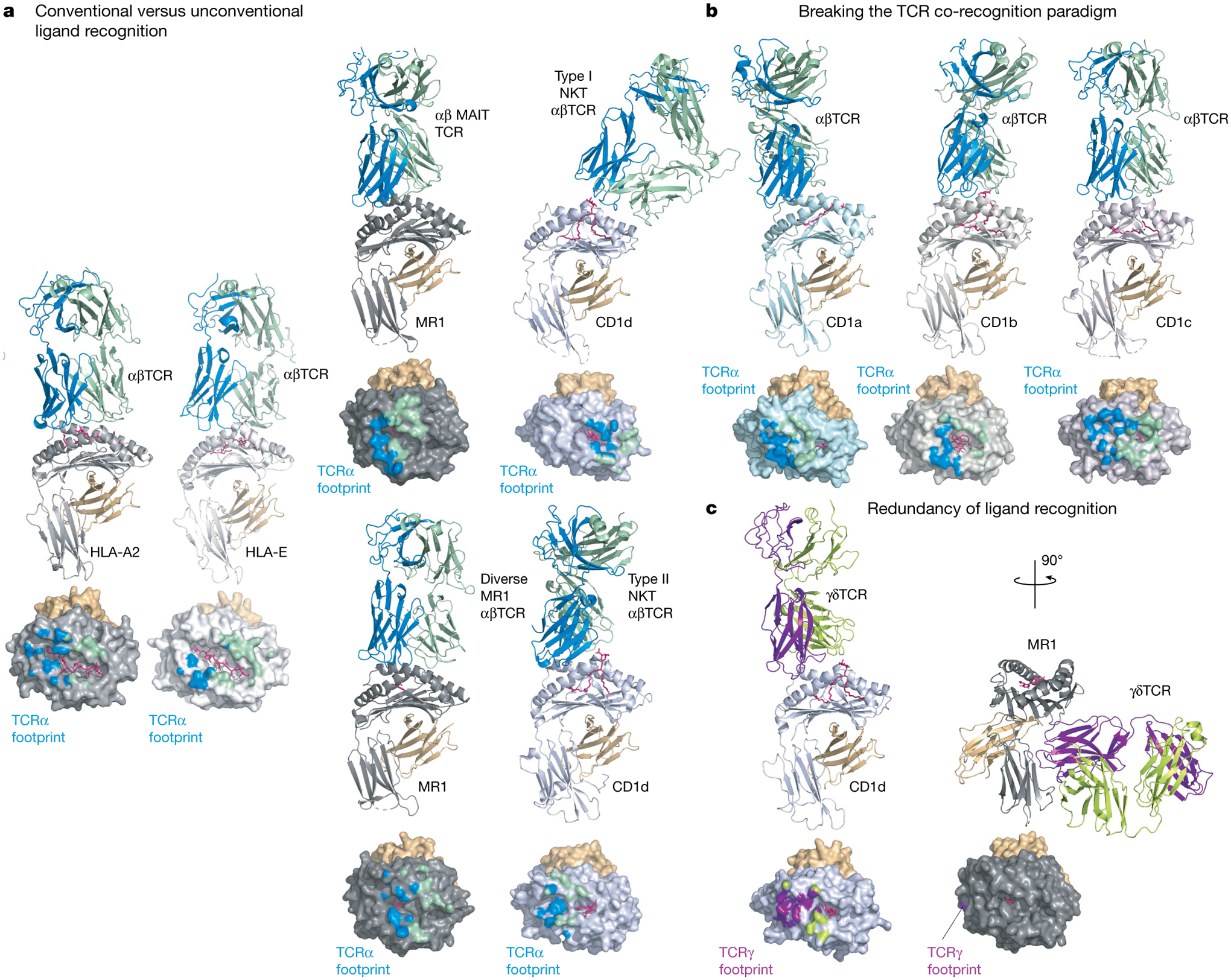
Experimentally determined TCR-binding modes are shown as cartoon representations for a selection of MHC class I or class I-like antigen-presenting molecules. In each panel the MHC-I subunit or equivalent is coloured as follows: light grey (HLA-A2), white (HLA-E), dark grey (MR1), light blue (CD1a), steel (CD1b), light pink (CD1c) or blue-white (CD1d). The respective antigens are coloured pink and associated β2-microglobulin orange; the interacting TCR subunits are coloured either blue (α-subunit) and green (β-subunit) or purple (δ-subunit) and lemon (γ-subunit). Below each structure is a surface representation of the antigen-presenting MHC-I molecules coloured according to their TCR subunit-recognition surfaces. a, Conventional versus unconventional ligand recognition. From left to right: a tumour-associated MART-peptide antigen in complex with HLA-A2 or HLA-E in complex with an αβTCR67,138; MR1-presenting vitamin metabolites recognized by a MAIT TCR70, a diverse αβTCR74 and CD1d in complex with type I139 and type II72 NKT TCRs. b, Breaking the TCR co-recognition paradigm. From left to right: an autoreactive αβTCR in complex with a self-lipid presented by CD1a25; CD1b in complex with a mycobacterial lipid recognized by a GEM TCR46; and an autoreactive TCR recognizing CD1c75. c, Redundancy of ligand recognition by alternative unconventional T cell subsets. The diversity of γδTCR recognition is shown with a CD1d-reactive γδTCR using a relatively standard docking mode55 (left) or the more radical recognition of the underside of MR1 (right)57.
