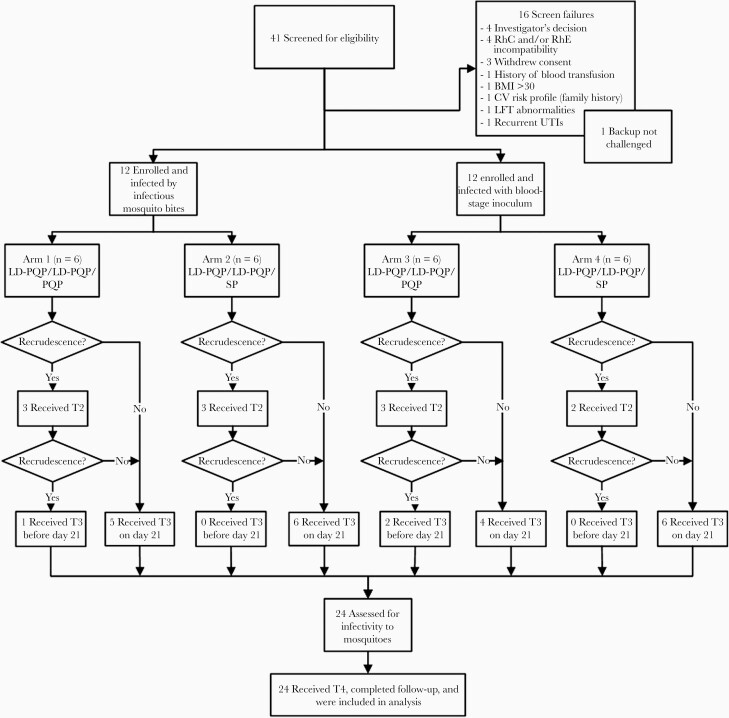
Figure 1.
Trial flow chart. Forty-one individuals were screened for eligibility, of whom 24 were included and divided over 4 study arms. All participants received low-dose piperaquine (LD-PQP) (480 mg) on day 8 (IBSM group) or when parasitemia reached a parasite count of 5000/mL (MB group) (first subcurative treatment [T1]. Participants received a second treatment with LD-PQP (second subcurative treatment [T2]) on recrudescence (parasites, 1500/mL) and a single, high-dose treatment (T3) on second recrudescence (1500/mL). Owing to thrombocytopenia, 1 participant in arm 1 received T1 7.5 days after inoculation and T3 on day 12.5. Because recrudescence occurred 15 days later, the final treatment (T4) with atovaquone-proguanil was initiated on day 27. Asexual recrudescence occurred in 2 IBSM participants after T1, and both received T3 directly (20.5 days after inoculation). All remaining participants of both cohorts received high-dose PQP (960 mg) or sulfadoxine-pyrimethamine (SP) (1000 mg/50 mg) (T3) on day 21 and final treatment with atovaquone-proguanil (T4) 36 days after inoculation. MB; mosquito bite. IBSM; induced blood-stage malaria. Abbreviations: BMI, body mass index; CV, cardiovascular; LFT, liver function test; UTIs, urinary tract infections.