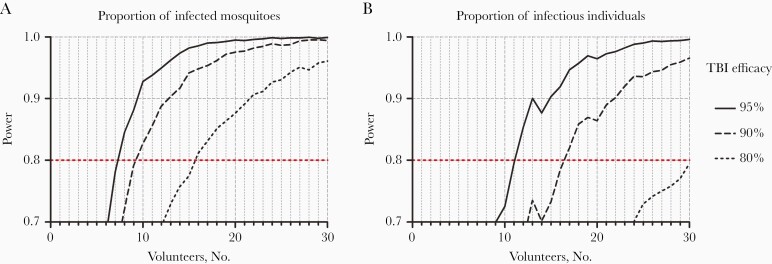
Figure 5.
Sample size requirements to use the current model to examine the efficacy of transmission-blocking interventions (TBIs). Sample size calculations were performed using the data from arm 3 of the current study (n = 6) and the controlled human malaria infection transmission trial conducted in Brisbane (n = 12) [10]. Three mosquito feeding time points per person were assumed. Power estimates are based on 4000 simulations per trial design, accounting for uncertainty in estimated transmission probability (log-odds, −3.64, 95% bayesian credible interval, −4.35 to −3.08) and interindividual variability (standard deviation on log-odds scale of 1.12; 95% bayesian credible interval, .59–1.93). Lines indicate TBI efficacies of 80%, 90%, and 95%, and power was calculated for comparisons of whether people were infectious (B; infecting ≥1 mosquito) and the proportion of mosquitoes they infected (A). Detecting a statistically significant difference in the proportion of infected mosquitoes between vaccinated and nonvaccinated participants with TBI efficacy of 95%, 90%, or 80% required 7, 10, or 15 volunteers per arm, respectively (80% power; P < .05).