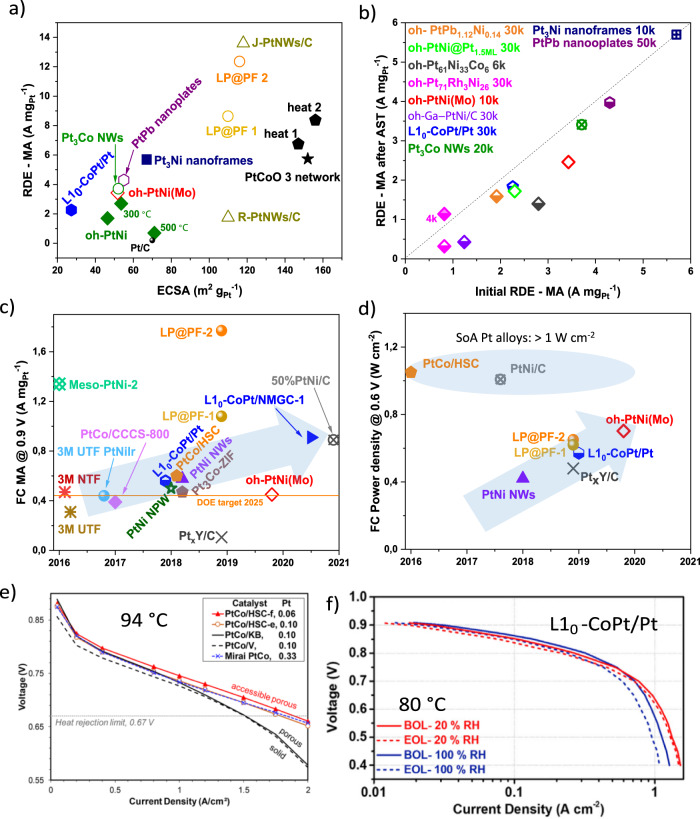
Fig. 3. Current performance of PGM catalysts.
a MAs at 0.9 VRHE as a function of ECSAs, and b MAs at 0.9 VRHE before and after a different number of potential cycles, from RDE measurements. In panel (a) the ECSA was obtained from either hydrogen underpotential deposition (Hupd, open symbols) or CO stripping (full colored symbols). c MAs at 0.9 V for low-PGM catalysts calculated from PEMFC performance with H2–O2, 1.0 bar, 80 °C, and 100 RH %, and d power density performance of promising low-PGM catalysts evaluated at 0.6 V in PEMFCs under hydrogen/air feeds at different loadings and conditions: 100% RH, 80 °C, stoichiometries are H2/Air 1.5/2 and absolute pressure of: For PtxY/C: 170 kPaabs. For PtNi/C and oh-PtNi(Mo): 100 kPag, inlet. For the remaining catalysts: 150 kPaabs. While SoA Pt alloys such as PtCo and PtNi show the highest power densities, promising catalysts with alternative designs have improved their performances over the recent years, as highlighted by the arrow in panel (d). Catalysts include PtxY/C117, PtNi NWs120, nanoframes26 and nanopinwheels (NPWs)121, Pt3Co NWs28, PtCo/HSC69,122, L10-CoPt27, L10-CoPt on hydrogel (L10-CoPt/NMGC-1)57, 50% PtNi/C56, PtNi/C123, PtPb nanoplates29, oh-PtNi@Pt1.5ML32, R-PtNWs and J-PtNWs37, Oh-PtNi (300 and 500)38, LP@PF17, PtCoO 3, heat 1, heat 274, Oh-PtNi(M), M = Mo23,44, Rh41, Ga43, Co124, and Pb125. 3M UTF PtNiIr126, Meso-PtNi-2127, 3M UTF and NTF126, PtCo/CCCS-80058 and Pt3Co-ZIF16. Panel b and c are adapted from ref. 2. e Air/H2 fuel cell performance of SoA PtCo catalysts on different carbons. Cathode Pt loadings in the legend, 0.25 mgPt cm−2 for the anode. Anode/cathode operating conditions: H2/air, 94 °C, 65/65%RH, 250/250 kPaabs,outlet, stoichiometries of 1.5/2, 50 cm2 active area69. f H2/air fuel cell polarization curves obtained with L10-CoPt/Pt catalysts, showing SoA mass activity in H2/O2 feed above 2025 US Department of Energy (DOE) target (cf. panel b). Conditions: 8 wt% Pt content, Pt loading of 0.105 mgPt cm−2, 80 °C, RH in the legend, 150 kPaabs H2/air, gas flow rate of 500/1000 sccm27. Panel e adapted with permission from ref. 69. Copyright 2018 American Chemical Society.