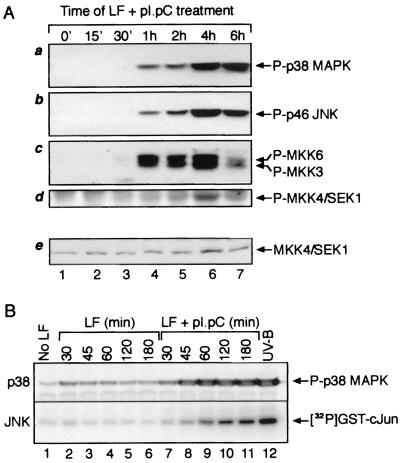
FIG. 1.
Phosphorylation of p38 MAPK, JNK, MKK3, MKK6, and SEK1/MKK4 and increased kinase activity of JNK in response to dsRNA. (A) Immunoblot analysis. HeLa cells were grown to ∼80% confluence in normal growth medium. The cells were then treated with pI · pC (3 μg/ml) in the presence of Lipofectin (LF; 10 μg/ml). At indicated times, the cells were harvested and cell lysates representing equal number of cells were subjected to immunoblot analyses with antibodies specific for the phosphorylated forms of p38 MAPK, JNK, MKK3, MKK6, and SEK1/MKK4 (see Materials and Methods). Panel e shows an immunoblot analysis of the levels of total (phosphorylated and nonphosphorylated) SEK1/MKK4 run in a parallel gel. (B) Analogous analysis of pI · pC action in Rat-1 fibroblasts, demonstrating also that pI · pC, and not Lipofectin, is the kinase-activating agent. A direct determination of JNK activity (rather than phosphorylation of JNK) after pI · pC treatment is presented. JNK1 was immunoprecipitated from Rat-1 cells, and the activity of the kinase was determined in immunocomplex kinase reactions using glutathione S-transferase–c-Jun as the substrate for phosphorylation (see Materials and Methods).