Abstract
Purpose
This study was designed to investigate the prognostic role of preoperative 68Ga-PSMA-11 PET/CT in predicting biochemical recurrence (BCR) of localized prostate cancer (PCa) after radical prostatectomy (RP).
Methods
A total of 77 biopsy-confirmed PCa patients with 68Ga-PSMA-11 PET/CT prior to RP were included. A PSMA-ligand PET/CT-based risk model with SUVmax, maximum diameter of the index tumor and T stage was developed for prediction of 2-year BCR using Cox regression analysis. Also, the efficacy of the developed risk model was compared with European Association of Urology risk stratification (D’Amico) and the Cancer of the Prostate Risk Assessment (CAPRA) score. C-index and calibration plot were used to assess discrimination and calibration with internal validation.
Results
With a median follow-up of 25 months, 23 (29.9%) patients experienced BCR within 2 years after RP. Patients experienced BCR had a significant higher PSA at diagnosis (p<0.001), a higher ISUP grade of biopsy (p=0.044), as well as a higher ISUP grade (p=0.001), a higher possibility of T3 diseases (p=0.001) and positive margin (p=0.008) on postoperative pathology. SUVmax, maximum diameter of the index tumor and T stage on preoperative PSMA-ligand PET/CT were significantly associated with BCR (all p<0.01). PSMA-ligand PET/CT-based risk model had a superior discrimination (c-index 78.5%) and good calibration at internal validation. The efficacy of this model in predicting 2-year BCR after RP was better, compared with CAPRA (c-index 66.3%) and D’Amico (c-index 66.2%). The addition of the PSMA-ligand PET/CT-derived variables also improved the efficacy of the existing models in predicting 2-year BCR (C-index of 78.9% for modified CAPRA and 79.3% for modified D’Amico, respectively).
Conclusion
A PSMA-ligand PET/CT-based risk model showed good efficacy in predicting 2-year BCR after RP, which needed to be validated by further prospective studies.
Keywords: prostate cancer, PSMA - prostate specific membrane antigen, radical prostatectomy, biochemical recurrence (BCR), prediction
Introduction
Radical prostatectomy (RP) is a widely adopted definitive option for men with localized prostate cancer (PCa) (1, 2). However, up to 40% of patients experienced biochemical recurrence (BCR) after RP (3). Several clinical models, such as D’Amico risk stratification scheme (4), and the University of California, San Francisco, Cancer of the Prostate Risk Assessment (CAPRA) score, have been developed to predict BCR (5). Preoperative variables such as prostate-specific antigen (PSA), clinical T staging, and Gleason score of systematic biopsy are used as prognostic factors in these models. However, the efficacy of these nomograms are far from excellent, with the prediction accuracy of 5-year BCR less than 70% (6, 7).
Prostate specific membrane antigen (PSMA)-ligand positron emission tomography/computed tomography (PET/CT) is currently a promising technique for recurrent PCa imaging (8, 9), as well as primary staging (10, 11). Our previous study indicates improved sensitivity of PSMA-ligand PET/CT in describing intraprostatic tumor lesions compared with multiparametric magnetic resonance Imaging (mpMRI) (12). In addition, increased PSMA uptake on PSMA-ligand PET/CT has been indicated to be positively correlates with prostate cancer aggressiveness and adverse pathologic features in our previous studies (13, 14), making PSMA-ligand PET/CT a potential tool to predict BCR following RP. Nonetheless, current models for prediction of BCR are mostly based on clinical and pathologic variables. The predictive role of PSMA-ligand PET/CT in this setting has been rarely investigated (15). Furthermore, the added value of PSMA-ligand PET/CT over the pre-existing models has not been evaluated.
Therefore, this study was designed to assess the potential role of PSMA-ligand PET/CT as a biomarker to predict early BCR after RP. We developed a PSMA-ligand PET/CT-based risk model for the prediction of BCR. The added value of PSMA-ligand PET/CT to the commonly used clinical models to predict BCR was also evaluated.
Patients and Methods
Study Population
We retrospectively included 138 consecutive patients with biopsy-confirmed prostate cancer who underwent 68Ga-PSMA-11 PET/CT before radical prostatectomy (RP) between January 2017 and June 2019. We excluded the patients with suspicious pelvic lymph nodes (n=11) or distant metastases (n=5). Patients who received treatment before RP (TURP, n=2; hormone therapy, n=35) were also excluded. Patients with inadequate clinical or pathological information (n=3) or incomplete follow-up information were also excluded (n=5). Finally, 77 patients were eligible for the analysis. This study was approved by the Ethics Committee of the Drum Tower Hospital (2017-147-01).
PSMA-Ligand PET/CT Scanning and Image Evaluation
68Ga-PSMA-11 PET/CT was acquired as previously described (12). 68Ga-PSMA-11 was synthesized using an ITG semiautomated module and were injected intravenously one hour before scanning. All PET/CT scans were performed in an uMI 780 PET/CT scanner (United Imaging Healthcare (UIH), Shanghai, China). A CT scan (130 keV, 80 mAs) and a static emission scans, corrected for dead time, scatter and decay, were acquired from the vertex to the proximal legs. PSMA-ligand PET/CT imaging were double reviewed by two experienced nuclear medicine physicians (SZ and SA). Lesions were delineate by higher uptake than background or blood pool. Semi-quantitative analysis of PSMA intensity was evaluated by an automated standard maximum uptake value (SUVmax) in the delineated lesion. For patients with multiple lesions, the one with highest SUVmax was recognized as the index tumor. The maximum diameter of the index tumor was also measured based on the delineate lesions previous recognized by nuclear medicine physicians on PET imaging as primary tumor is not distinctly visible on CT alone. For the assessment of T stage on PSMA-ligand PET/CT, all the assessment were based on the fusion image of PET and CT. PET image with angulated contour of the prostate gland or obliteration of the recto-prostatic angle accordant with the shape on CT were recognized as extracapsular extension (T3a) while seminal vesicle invasion (T3b) was diagnosed if there is a focal or diffuse 68Ga-PSMA-11 accumulation above the background (16).
Covariates, Endpoints, and Model Development
Clinical information including age, PSA level at diagnosis and clinical stage assessed by digital rectal examination (DRE) were included. Transperineal systematic prostate biopsy were performed, with additional fusion targeted biopsies if suspicious lesions (PI-RADS 3-5) were detected on multiparametric magnetic resonance imaging (mpMRI). For preoperative parameters of biopsy, Gleason score and percentage of positive cores were collected. For, PSMA-ligand PET/CT-derived parameters, we included SUVmax, maximum diameter of the index tumor, and T stage. Postoperative BCR was defined as three successive rises in PSA level of >0.1 ng/ml at least 6 weeks postoperatively with final PSA >0.2 ng/ml (n=19), or administration of secondary therapy for evidence of detectable PSA >0.1 ng/ml at least 6 weeks postoperatively (n=10) (17).
PSMA-ligand PET/CT-based model was developed by inputting PSMA-ligand PET/CT-derived variables (SUVmax, maximum diameter of the index tumor, and T stage). For the existing clinical models, D’Amico and CAPRA scores were collected according to the established D’Amico and CAPRA risk stratification scheme (5), by inputting clinical variables such as patient age, PSA level at diagnosis, Gleason score at biopsy, percentage of positive cores at biopsy, and clinical T stage assessed by DRE. To investigate the added value of PSMA-ligand PET/CT-derived parameters to the existing clinical models, modified D’Amico and modified CAPRA were developed. D’Amico score or CAPRA score was integrated with SUVmax, maximum diameter and T stage and re-assessed by Cox regression analyses.
Statistical Analysis
Mann-Whitney U test was performed for continuous variables and the Fisher exact test/chi-square test for categorical variables to compare the characteristics between the patients who underwent BCR and those free from BCR at 2-year follow-up. The risk of BCR was predicted using Cox regression model. By plotting the observed versus predicted cumulative incidences within 2 years after RP, we also assessed the calibration of our risk model. The discrimination of our risk model and modified D’Amico or CAPRA models was assessed by the concordance index (C-Index). The C-index and calibration plots were produced using the predicted probabilities after a validation with bootstrap by 1000 iterations. A significance level of 5% was used. All analyses were performed using SPSS software, version 22.0 (IBM Corp.) and R statistical package v.3.0.2 (R Project for Statistical Computing, www.r-project.org).
Results
Patient Characteristics and Survival Analysis
Table 1 showed the clinical, preoperative and postoperative pathological characteristics as well as the PSMA-ligand PET/CT-derived features of the 77 patients, with a median age of 69 (interquartile range [IQR]: 62–73 years and median PSA 13.30 ng/ml (IQR: 7.89-28.70) at diagnosis. The median (IQR) follow-up time were 25 (19-27) months for all patients, 25 (21.5-26.8) months for BCR patients and 26 (17.5-27) months for BCR-free patients. Twenty-nine (37.7%) and 23 (29.9%) patients experienced BCR overall and within 24 months after RP. The patients were divided into two groups according to the status of BCR at 2-year follow-up. All clinical, pathological, and imaging variables were compared between the two groups.
Table 1.
Characteristics of prostate cancer patients with 68Ga-PSMA PET/CT scanning prior to radical prostatectomy.
| Characteristics | Total (n = 77) | Median (IQR) or n (%) | p | |
|---|---|---|---|---|
| BCR Free (n = 54) | BCR (n = 23) | |||
| Preoperative characteristics | ||||
| Age | 69 (65-75) | 69 (65-74) | 68 (65.5-75.5) | 0.993 |
| PSA | 13.30 (7.89-28.70) | 10.89 (6.61-16.00) | 32.25 (14.05-71.43) | 0.000 |
| Clinical T stage by DRE | 0.356 | |||
| T2 | 71 (92.2) | 51 (94.4) | 20 (87.0) | |
| T3 | 6 (7.8) | 3 (5.6) | 3 (13.0) | |
| ISUP at Biopsy | 0.044 | |||
| 1 | 14 (18.2) | 13 (24.1) | 1 (4.3) | |
| 2 | 18 (23.4) | 13 (24.1) | 5 (21.7) | |
| 3 | 16 (20.8) | 13 (24.1) | 3 (13.0) | |
| 4 | 21 (27.3) | 10 (18.5) | 11 (47.8) | |
| 5 | 8 (10.4) | 5 (9.3) | 3 (13.0) | |
| Percent of positive cores on biopsy | 35.71 (21.42-55.91) | 30.0 (21.4-51.6) | 42.9 (28.1-57.64) | 0.130 |
| Postoperative characteristics | ||||
| Post-operative ISUP | 0.001 | |||
| 1 | 5 (6.5) | 5 (9.3) | 0 (0) | |
| 2 | 25 (32.5) | 22 (40.7) | 3 (13.0) | |
| 3 | 18 (23.4) | 15 (27.8) | 3 (13.0) | |
| 4 | 16 (20.8) | 7 (13.0) | 9 (39.1) | |
| 5 | 13 (16.9) | 5 (9.3) | 8 (34.8) | |
| Pathological T stage, n (%) | 0.001 | |||
| T2 | 27 (35.5) | 23 (43.4) | 4 (17.4) | |
| T3a | 35 (46.1) | 26 (49.1) | 9 (39.1) | |
| T3b | 14 (18.4) | 4 (7.5) | 10 (43.5) | |
| Positive margin | 0.008 | |||
| Absent | 56 (72.7) | 12 (52.2) | 44 (81.5) | |
| Present | 21 (27.3) | 11 (47.8) | 10 (18.5) | |
| Preoperative PET/CT features | ||||
| SUVmax | 13.04 (7.76-21.60) | 10.70 (6.83-17.00) | 22.90 (15.74-31.01) | 0.000 |
| Maximum diameter (cm) | 1.19 (0.76-2.27) | 1.09 (0.74-1.80) | 1.93 (1.13-2.44) | 0.008 |
| PET-detected T stage | 0.002 | |||
| T2 | 46 (59.7) | 39 (72.2) | 7 (30.4) | |
| T3a | 23 (29.9) | 12 (22.2) | 11 (47.8) | |
| T3b | 8 (10.4) | 3 (5.6) | 5 (21.7) | |
PSMA, prostate specific membrane antigen; PET/CT, positron emission computed tomography; IQR, interquartile range; BCR, biochemical recurrence; PSA, prostate specific antigen; DRE, digital rectal examination; SUV, standard uptake value; ISUP, International Society of Urological Pathology; cm, centimeter.
Significant P values were presented in bold text.
At the time point of 24-month follow-up, 23 (29.9%) had experienced BCR while the other 54 (70.1%) are free from BCR. Table 1 showed summary characteristics of the two groups. The BCR group had a significantly higher PSA level at diagnosis (32.25 versus 10.89 ng/ml), a higher ISUP grade of biopsy (p=0.044), as well as a higher ISUP grade (p=0.001), a higher possibility of T3 diseases (p=0.001) and positive margin (p=0.008) on postoperative pathology. For parameters on PSMA-ligand PET/CT, patients with BCR had a higher SUVmax, a larger maximum diameters and a higher T stage than BCR-free patients.
Multivariable Models Predicting BCR
In Cox regression, PSMA-ligand PET/CT-based model with input of SUVmax, maximum diameters, and T stage on PSMA-ligand PET/CT achieved a superior discrimination of BCR during the 2-year follow-up than CAPRA [C-Index: 78.5% (70.3-86.7%) versus 66.3 (76.5-56.1)] and D’Amico [C-Index: 78.5% (70.3-86.7%) versus 66.2 (75.0-57.4)] ( Table 2 ). This model was also characterized by a good calibration at internal validation ( Figure 1 ). The inclusion of the PSMA-ligand PET/CT-derived variables also improved the efficacy of the existing models in predicting post-surgery BCR (C-Index: 66.3 versus 78.9% for CAPRA and modified CAPRA; C-Index: 66.2 versus 79.3 for D’Amico and modified D’Amico) ( Table 2 ).
Table 2.
Cox regression analyses assessing the prediction models of biochemical recurrence in prostate cancer patients treated with radical prostatectomy.
| Parameters | PET/CT based risk model | D’Amico | CAPRA | Modified D’Amico | Modified CAPRA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| PET/CT T stage | ||||||||||
| T2 | 1 (ref) | - | - | - | - | 1 (ref) | 1 (ref) | |||
| T3a | 2.92 (1.08-7.87) | .034 | - | - | - | - | 2.64 (0.38-7.15) | .055 | 2.76 (1.02-7.47) | .045 |
| T3b | 2.29 (0.54-9.72) | .260 | - | - | - | - | 1.74 (0.57-7.29) | .449 | 1.99 (0.50-8.61) | .358 |
| SUVmax | 1.04 (1.02-1.07) | .002 | - | - | - | - | 1.04 (0.96-1.07) | .003 | 1.04 (1.01-1.07) | .004 |
| Maximum diameter on PET | 0.97 (0.58-1.61) | .905 | - | - | - | - | 0.88 (1.14-1.51) | .632 | 0.90 (0.59-1.56) | .686 |
| D’Amico score | - | - | 2.71 (1.37-5.38) | 0.004 | - | - | 2.01 (0.50-4.41) | .083 | - | - |
| CAPRA score | - | - | - | - | 1.30 (1.09-1.56) | 0.004 | - | - | 1.11 (0.90-1.38) | .363 |
| C-Index | 78.5 (70.3-86.7) | 66.2 (57.4-75.0) | 66.3 (56.1-76.5) | 79.3 (70.1-88.5) | 78.9 (70.4-87.3) | |||||
PET/CT, positron emission computed tomography; HR, hazard ratio; CI, confidence intervals; SUV, standard uptake value.
Significant p values were presented in bold text.
Figure 1.
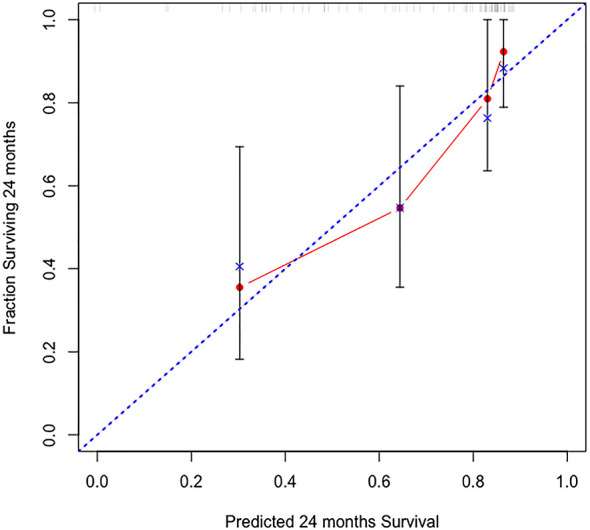
Calibration plot of observed proportion versus predicted probability of 2-year biochemical recurrence after radical prostatectomy by the PSMA-ligand PET/CT-based risk model.
Discussion
The newly developed risk model based on 68Ga-PSMA-11 PET/CT-derived parameters (SUVmax, maximal diameter of index tumor, and T staging) showed better performance in predicting 2-year BCR, compared with that of D’Amico and CAPRA models. Furthermore, we found that addition of parameters obtained from PSMA-ligand PET/CT outperformed models based on clinical and biopsy variables. To the best of our knowledge, this was the first study to develop a PSMA-ligand PET/CT-based model for prediction of BCR after RP.
The evidence investigating the role of PSMA-ligand PET/CT in predicting BCR after RP was very limited. Roberts et al. showed that intraprostatic 68Ga-PSMA-11 intensity (SUVmax) was one of the significant pre-operative predictors of progression-free survival after RP. Sub-analysis indicated that SUVmax was the most significant predictor of progression-free survival in patients with biopsy Gleason score ≤ 4 + 3 (15). Our study developed a new risk model only based on parameters derived from PSMA-ligand PET/CT, which was different from Roberts’s study that SUVmax was added to clinical and pathological variables for Cox regression analysis. In addition, the comparison of the efficacy to the existing clinical models was not performed in Roberts’s study.
In the present study, our risk model showed better performance compared to the commonly used D’Amico and CAPRA models ( Table 2 ), although only three parameters (SUVmax, T staging, and tumor size described on PSMA PET/CT) were included. This result might be explained by the better performance of PSMA-ligand PET/CT-derived parameters in indicating histopathological features compared with clinical parameters. The most commonly used clinical variable reflecting tumor aggressiveness was PSA. However, PSA was an organ-specific biomarker instead of a disease-specific biomarker (18), as it could be induced to be released by several benign diseases such as benign prostatic hyperplasia (BPH), and prostatitis (19). In contrast, PSMA could be considered as PCa-specific marker, as it was highly expressed on the surface of PCa cells (20, 21). The other clinical variable that reflected tumor aggressiveness was histopathology obtained from prostate biopsy. However, Gleason score of prostate biopsy was always related with underestimation of tumor aggressiveness, as Gleason score upgrading from systematic biopsy to RP was commonly reported (22). Though MRI-targeted biopsy increased the detection rate of clinical significant PCa, it was associated with a 30.9% upgrading of cancer group (23). It might be due to the relatively low sensitivity of mpMRI in detecting intraprostatic lesions, especially for small lesions with low grade (24). Moreover, only small part of tissue was obtained from targeted biopsy, which was difficult to reflect the tumor grade of the whole lesion. It has been demonstrated that the detection rate was improved when the number of targeted biopsy increased (25). Different from biopsy, preoperative PSMA-ligand PET/CT was more informative for tumor grade reporting. Previous studies had revealed that SUV derived from PSMA PET/CT was positively correlated with tumor Gleason score (26, 27).
T staging on PSMA-ligand PET/CT is another contributor for better performance of our risk model in predicting of BCR compared with clinical models ( Table 2 ). Clinically, tumor staging is assessed by DRE. Apparently, PSMA-ligand PET/CT provides more precise information regarding tumor size and tumor location compared with DRE, improving the efficacy of PSMA-ligand PET/CT in evaluating clinical staging of T1 and T2. Recently, accumulative evidence shows the equivalent and even improved efficacy of PSMA-ligand PET/CT in detecting extraprostatic extension (EPE) and seminal vesical invasion (SVI) compared with mpMRI (16, 28), which could explain the significantly improved efficacy of PSMA-ligand PET/CT in providing tumor information regarding T3 staging. As shown in Table 2 , T3a on PSMA-ligand PET/CT was significantly associated with the higher BCR after RP. However, this was not observed in patients with T3b, which has been reported to be a strong risk factor for BCR. It might be due to the smaller sample size in this polit study. Only 8 patients (10.4%) ( Table 1 ) with T3b staging on PSMA-ligand PET/CT were included in the present study.
In our study, about 30% (23/77) patients experienced BCR within 2-year follow-up post PR, which was higher than the published results (29). Since tumor grade had been well demonstrated to be an independent predictor for early BCR after RP (5), our results could be explained by more cases with higher Gleason score (preoperative ISUP>2: 58.5% versus 36%) (30). Also, the median PSA level in the present study was much higher compared with that in the published study (13.30 versus 7.49 ng/ml) (30). Different from the United States, PCa screening was less pervasive in China, resulting in much higher percentage of high/very high risk and even metastatic patients at initial diagnosis (31). Therefore, the efficacy of our risk model for low-to-intermediate risk cases needed to be further validated with external data.
Regarding limitations, our study was a single-center retrospective study with relatively small sample and the median follow-up was only 25 months for patients without BCR. Therefore, our model needed to be further validated on patients with a longer follow-up procedure, as they might experience BCR after maintaining BCR-free survival within this period. To avoid selection bias, our risk model needed to be validated by further prospective studies before clinical application, as patients with pelvic lymph nodes and distant metastases were not included. However, our study aimed to propose the perspective that PSMA-ligand-based risk model might have great potential for risk stratification and prediction of BCR after RP, as it could provide noninvasive and prospective information regarding tumor aggressiveness and prognosis. In our established model, the weight of maximal tumor diameters seemed to be limited compared with T stage and SUVmax, though there was a significant difference between the BCR-free patients and BCT patients. In addition, the measurement of the maximal diameters on PET/CT is tricky though all measurements in the present study were performed by the same team with the same methods. Therefore, the value of maximal tumor diameters needs to be further verified and optimized in the following study.
In conclusion, a PSMA-ligand PET/CT-based risk model was developed for the BCR prediction following RP. Our newly developed risk model was shown to have better efficacy in predicting 2-year BCR after RP than the current D’Amico and CAPRA nomograms. Furthermore, the efficacy of the existing models were significantly improved by the additions of the parameters derived from PSMA-ligand PET/CT. PSMA-ligand PET/CT-based risk model showed great potential for the risk stratification and prediction of BCR of localized PCa after RP, which needed to be further validation by prospective studies.
Conclusion
This study demonstrated that a PSMA-ligand PET/CT based model had a good efficacy in predicting 2-year BCR after RP and the efficacy of the existing models were significantly improved by the additions of the parameters derived from PSMA-ligand PET/CT.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by 2017-147-01. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81602232, 81802535), Nanjing Medical Science and technique Development Foundation (QRX17128), and Nanjing Health Distinguished Youth Fund (JQX16025). All the funding supported equally in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Network JNCCN (2019) 17:479–505. doi: 10.6004/jnccn.2019.0023 [DOI] [PubMed] [Google Scholar]
- 2. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment With Curative Intent. Eur Urol (2017) 71:618–29. doi: 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 3. Babaian RJ, Troncoso P, Bhadkamkar VA, Johnston DA. Analysis of Clinicopathologic Factors Predicting Outcome After Radical Prostatectomy. Cancer (2001) 91:1414–22. doi: [DOI] [PubMed] [Google Scholar]
- 4. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical Outcome After Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA (1998) 280:969–74. doi: 10.1001/jama.280.11.969 [DOI] [PubMed] [Google Scholar]
- 5. Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment Score: A Straightforward and Reliable Preoperative Predictor of Disease Recurrence After Radical Prostatectomy. J Urol (2005) 173:1938–42. doi: 10.1097/01.ju.0000158155.33890.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lughezzani G, Budäus L, Isbarn H, Sun M, Perrotte P, Haese A, et al. Head-To-Head Comparison of the Three Most Commonly Used Preoperative Models for Prediction of Biochemical Recurrence After Radical Prostatectomy. Eur Urol (2010) 57:562–8. doi: 10.1016/j.eururo.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 7. Morote J, Del Amo J, Borque A, Ars E, Hernández C, Herranz F, et al. Improved Prediction of Biochemical Recurrence After Radical Prostatectomy by Genetic Polymorphisms. J Urol (2010) 184:506–11. doi: 10.1016/j.juro.2010.03.144 [DOI] [PubMed] [Google Scholar]
- 8. Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic Performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in Patients With Recurrent Prostate Cancer: Evaluation in 1007 Patients. Eur J Nucl Med Mol Imaging (2017) 44:1258–68. doi: 10.1007/s00259-017-3711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rauscher I, Düwel C, Haller B, Rischpler C, Heck MM, Gschwend JE, et al. Efficacy, Predictive Factors, and Prediction Nomograms for (68)Ga-Labeled Prostate-Specific Membrane Antigen-Ligand Positron-Emission Tomography/Computed Tomography in Early Biochemical Recurrent Prostate Cancer After Radical Prostatectomy. Eur Urol (2018) 73:656–61. doi: 10.1016/j.eururo.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 10. Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, et al. (68)Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients With Prostate Cancer. Eur Urol (2016) 70:553–7. doi: 10.1016/j.eururo.2015.12.051 [DOI] [PubMed] [Google Scholar]
- 11. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-Specific Membrane Antigen PET-CT in Patients With High-Risk Prostate Cancer Before Curative-Intent Surgery or Radiotherapy (proPSMA): A Prospective, Randomised, Multicentre Study. Lancet (Lond Engl) (2020) 395:1208–16. doi: 10.1016/S0140-6736(20)30314-7 [DOI] [PubMed] [Google Scholar]
- 12. Chen M, Zhang Q, Zhang C, Zhao X, Marra G, Gao J, et al. Combination of (68)Ga-PSMA PET/CT and Multiparametric MRI Improves the Detection of Clinically Significant Prostate Cancer: A Lesion-By-Lesion Analysis. J Nucl Med Off Publication Soc Nucl Med (2019) 60:944–9. doi: 10.2967/jnumed.118.221010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen M, Qiu X, Zhang Q, Zhang C, Zhou Y, Zhao X, et al. PSMA Uptake on [68Ga]-PSMA-11-PET/CT Positively Corrects With Prostate Cancer Aggressiveness. Q J Nucl Med Off Publ Ital Assoc Nucl Med (AIMN) Int Assoc Radiopharm (IAR) (2019). doi: 10.1097/01.JU.0000557493.33637.42 [DOI] [PubMed] [Google Scholar]
- 14. Chen M, Zhang Q, Zhang C, Zhou YH, Zhao X, Fu Y, et al. Comparison of (68)Ga-Prostate-Specific Membrane Antigen (PSMA) Positron Emission Tomography/Computed Tomography (PET/CT) and Multi-Parametric Magnetic Resonance Imaging (MRI) in the Evaluation of Tumor Extension of Primary Prostate Cancer. Trans Androl Urol (2020) 9:382–90. doi: 10.21037/tau.2020.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts MJ, Morton A, Donato P, Kyle S, Pattison DA, Thomas P, et al. (68)Ga-PSMA PET/CT Tumour Intensity Pre-Operatively Predicts Adverse Pathological Outcomes and Progression-Free Survival in Localised Prostate Cancer. Eur J Nucl Med Mol Imaging (2021) 48(2):477–82. doi: 10.1007/s00259-020-04983-9 [DOI] [PubMed] [Google Scholar]
- 16. von Klot CJ, Merseburger AS, Böker A, Schmuck S, Ross TL, Bengel FM, et al. (68)Ga-PSMA PET/CT Imaging Predicting Intraprostatic Tumor Extent, Extracapsular Extension and Seminal Vesicle Invasion Prior to Radical Prostatectomy in Patients With Prostate Cancer. Nucl Med Mol Imaging (2017) 51:314–22. doi: 10.1007/s13139-017-0476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brockman JA, Alanee S, Vickers AJ, Scardino PT, Wood DP, Kibel AS, et al. Nomogram Predicting Prostate Cancer-Specific Mortality for Men With Biochemical Recurrence After Radical Prostatectomy. Eur Urol (2015) 67:1160–7. doi: 10.1016/j.eururo.2014.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balk SP, Ko YJ, Bubley GJ. Biology of Prostate-Specific Antigen. J Clin Oncol Off J Am Soc Clin Oncol (2003) 21:383–91. doi: 10.1200/JCO.2003.02.083 [DOI] [PubMed] [Google Scholar]
- 19. Gjertson CK, Albertsen PC. Use and Assessment of PSA in Prostate Cancer. Med Clinics North America (2011) 95:191–200. doi: 10.1016/j.mcna.2010.08.024 [DOI] [PubMed] [Google Scholar]
- 20. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-Specific Membrane Antigen Expression in Normal and Malignant Human Tissues. Clin Cancer Res an Off J Am Assoc Cancer Res (1997) 3:81–5. [PubMed] [Google Scholar]
- 21. Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate Specific Membrane Antigen Expression in Prostatic Intraepithelial Neoplasia and Adenocarcinoma: A Study of 184 Cases. Cancer (1998) 82:2256–61. doi: [DOI] [PubMed] [Google Scholar]
- 22. Hsieh TF, Chang CH, Chen WC, Chou CL, Chen CC, Wu HC. Correlation of Gleason Scores Between Needle-Core Biopsy and Radical Prostatectomy Specimens in Patients With Prostate Cancer. J Chin Med Assoc JCMA (2005) 68:167–71. doi: 10.1016/S1726-4901(09)70243-6 [DOI] [PubMed] [Google Scholar]
- 23. Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. New Engl J Med (2020) 382:917–28. doi: 10.1056/NEJMoa1910038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schouten MG, van der Leest M, Pokorny M, Hoogenboom M, Barentsz JO, Thompson LC, et al. Why and Where do We Miss Significant Prostate Cancer With Multi-Parametric Magnetic Resonance Imaging Followed by Magnetic Resonance-Guided and Transrectal Ultrasound-Guided Biopsy in Biopsy-Naïve Men? Eur Urol (2017) 71:896–903. doi: 10.1016/j.eururo.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 25. Stewart CS, Leibovich BC, Weaver AL, Lieber MM. Prostate Cancer Diagnosis Using a Saturation Needle Biopsy Technique After Previous Negative Sextant Biopsies. J Urol (2001) 166:86–91; discussion -2. doi: 10.1016/S0022-5347(05)66083-1 [DOI] [PubMed] [Google Scholar]
- 26. Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. (68)Ga-PSMA-11 PET/CT in Primary Staging of Prostate Cancer: PSA and Gleason Score Predict the Intensity of Tracer Accumulation in the Primary Tumour. Eur J Nucl Med Mol Imaging (2017) 44:941–9. doi: 10.1007/s00259-017-3631-6 [DOI] [PubMed] [Google Scholar]
- 27. Koerber SA, Utzinger MT, Kratochwil C, Kesch C, Haefner MF, Katayama S, et al. (68)Ga-PSMA-11 PET/CT in Newly Diagnosed Carcinoma of the Prostate: Correlation of Intraprostatic PSMA Uptake With Several Clinical Parameters. J Nucl Med Off Publication Soc Nucl Med (2017) 58:1943–8. doi: 10.2967/jnumed.117.190314 [DOI] [PubMed] [Google Scholar]
- 28. Grubmüller B, Baltzer P, Hartenbach S, D'Andrea D, Helbich TH, Haug AR, et al. PSMA Ligand PET/MRI for Primary Prostate Cancer: Staging Performance and Clinical Impact. Clin Cancer Res (2018) 24:6300–7. doi: 10.1158/1078-0432.CCR-18-0768 [DOI] [PubMed] [Google Scholar]
- 29. Coughlin GD, Yaxley JW, Chambers SK, Occhipinti S, Samaratunga H, Zajdlewicz L, et al. Robot-Assisted Laparoscopic Prostatectomy Versus Open Radical Retropubic Prostatectomy: 24-Month Outcomes From a Randomised Controlled Study. Lancet Oncol (2018) 19:1051–60. doi: 10.1016/S1470-2045(18)30357-7 [DOI] [PubMed] [Google Scholar]
- 30. Yaxley JW, Coughlin GD, Chambers SK, Occhipinti S, Samaratunga H, Zajdlewicz L, et al. Robot-Assisted Laparoscopic Prostatectomy Versus Open Radical Retropubic Prostatectomy: Early Outcomes From a Randomised Controlled Phase 3 Study. Lancet (Lond Engl) (2016) 388:1057–66. doi: 10.1016/S0140-6736(16)30592-X [DOI] [PubMed] [Google Scholar]
- 31. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA: Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.


