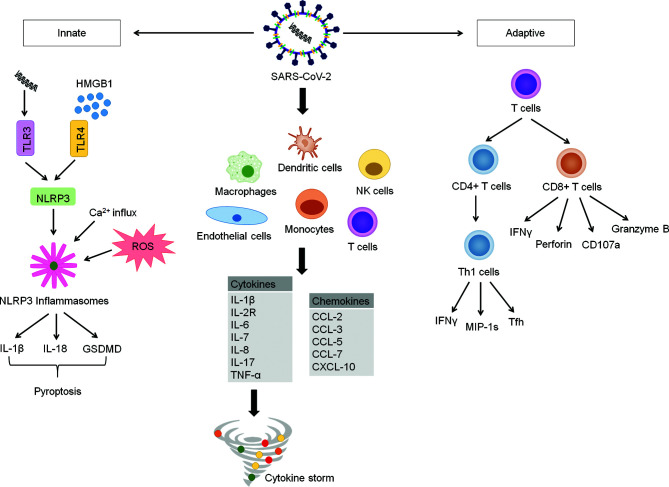
Figure 1.
COVID-19 Inflammatory Signaling Pathway. COVID-19 is characterized by delayed intracellular innate immune responses associated with type I and type III IFNs. This allows for rapid virus replication and subsequent PRR-induced abnormal inflammatory response. SARS-CoV-2 invade and activate of various immune cells such as endothelial cells, macrophages, monocytes, dendritic cells, natural killer cells and T-cells. These abnormally activated immune cells stimulate multiple inflammatory pathways producing large amounts of cytokines and chemokines, which results in hyperinflammation and cytokine storm. The formation of cytokine storm reduces further spread of virus in the body but also causes tissues damage, causing acute respiratory distress syndrome (ARDS) and multi-organ failure. Upon entering cells, SARS-COV-2 is recognized by pattern-recognition receptor (toll-like receptor 3), resulting in activation of NLRP3. Simultaneously, the viral invasion causes protein aggregation, calcium influx and ROS formation. These events lead to the initiation of NLRP3 inflammasome. Activated NLRP3 inflammasome stimulates activation of caspase-1, which cleaves Gasdermin D (GSDMD), resulting in pyroptosis of cells. Activated caspase-1 also promotes the secretion of IL-1β, IL-18, which play an important role in pyroptosis procession. HMGB1, a prototypical DAMP activates inflammasome and play an essential role in the inflammatory response in COVID-19 patients. The adaptive immunity kicks off soon after the trigger of innate immune response to control virus infection through antibody-producing B cells, CD4+ T cells and CD8+ T cells. CD4+ T cells are important for virus clearance, while CD8+ T cells provide protection against the virus through multiple cytokines. However, CD4+ and CD8+ T cells are found to be significantly reduced in patients with severe COVID-19.