Graphical abstract
Keywords: Endocrine disruption, Molecular docking, Non-covalent interaction, Nuclear receptor, Polychlorinated dibenzofurans
Highlights
-
•
Susceptibility of 12 nuclear receptors to binding by PCDFs was investigated in silico.
-
•
Androgen receptor was found to be susceptible to binding by PCDFs.
-
•
Male reproductive dysfunctions from PCDF exposure may be due to inappropriate binding of PCDFs to androgen receptor.
Abstract
Polychlorinated dibenzofurans (PCDFs) are known to cause endocrine disruption in humans and wildlife but the mechanisms underlying this disruption have not been adequately investigated. In this paper, the susceptibility of the endocrine system to disruption by PCDF congeners via nuclear receptor binding was studied using molecular docking simulation. Findings revealed that some PCDF congeners exhibit high probabilities of binding to androgen receptor in its agonistic and antagonistic conformations. In depth molecular docking analysis of the receptor-ligand complexes formed by PCDFs with androgen receptor in its agonistic and antagonistic conformations showed that, these complexes were stabilized by electrostatic, van der Waals, pi-effect and hydrophobic interactions. It was also observed that PCDF molecules mimic the modes of interaction observed in androgen-testosterone and androgen-bicalutamide complexes, utilizing between 65 and 83% of the amino acid residues used by the co-crystallized ligands for binding. This computational study suggests that some PCDF congeners may act as agonists and antagonists of androgen receptor in humans and wildlife via inapproprate binding to the receptor.
1. Introduction
There is an upsurge of interest and concern among scientists and the general public about the possible roles of some natural and anthropogenic chemicals in endocrine-related diseases. These chemicals, now known as endocrine-disrupting chemicals (EDCs), are common chemicals in the environment, foods, industrial products, cosmetics, and other personal care products (Caliman and Gavrilescu, 2009, Zhang et al., 2012, Muncke, 2009, Koo and Lee, 2004, Rudel et al., 2003, Darbre, 2018, Pojana et al., 2007, Rhind et al., 2013, Archer et al., 2017, Loffredo and Senesi, 2006). Although most of the chemicals now classified as EDCs were previously unknown to cause any deleterious effects in humans and wildlife, mounting evidence from clinical, epidemiological and experimental investigations carried out within the last three decades indicates that EDCs can cause deviation from normal homeostatic control, reproduction and developmental processes in humans and wildlife (Diamanti-Kandarakis et al., 2009, Darbre and Darbre, 2015, Gore et al., 2015). Specifically, exposure of wildlife to EDCs was observed to result in feminization of male fish, eggshell thinning in birds, and formation of imposex in mollusks (Darbre and Darbre, 2015). Human exposure to EDCs has also been shown in several studies to be associated with reduced semen quality, urogenital tract abnormalities, prostate cancer, precocious and early puberty, increased rate of ectopic pregnancy, early menopause, high risk of infertility, emergence of rare cervicovaginal cancer, increased risk of breast cancer, alteration of immune responses, neurobehavioral deficits, type-2 diabetes, obesity, and cardiovascular diseases (Miodovnik et al., 2011, Schug et al., 2011, Pinson et al., 2016, Rogers et al., 2013, Wang et al., 2016, Zamkowska et al., 2018, Hu et al., 2012, Hess-Wilson and Knudsen, 2006, Soto and Sonnenschein, 2010, Fernandez et al., 2012, Yum et al., 2013, Grindler et al., 2015, Khan and Zhang, 2019, Alonso-Magdalena et al., 2011, Newbold et al., 2008, Hatch et al., 2010).
Over the past 30 years, a great deal of research efforts was expended towards the identification of chemicals that act as EDCs and these efforts led to the discovery of several endocrine active substances. Chemical compounds suspected or identified to act as endocrine disruptors include phthalates (Feige et al., 2007, Hao et al., 2012), bisphenols (Molina-Molina et al., 2013, Rubin, 2011, Matsushima et al., 2007), parabens (Vitku et al., 2018, Kolatorova et al., 2018), triclosan (Raut and Angus, 2010, Foran et al., 2000), polyhalogenated aromatic organic pollutants (Aoki, 2001, Iwasaki et al., 2002, Goldey et al., 1995, Fernandez et al., 2010, Ünüvar and Büyükgebiz, 2012, Li et al., 2013, Yue et al., 2020), phytoestrogens (Santti et al., 1998), mycotoxins (Zielonka et al., 2015, Vejdovszky et al., 2017, El. Khoury et al., 2019), pesticides (Combarnous, 2017, Leemans et al., 2019), and some pharmaceuticals (Caliman and Gavrilescu, 2009). In recent times however, the direction of research is being shifted from mere EDC identification to studies that focus on understanding the underlying mechanisms by which individual EDC exerts its actions in humans and wildlife. The consensus of opinion among experts, according to La Merril et al. (La Merrill et al., 2020), reveals that EDCs possess the following ten identifiable key characteristics which are related to how they interfere with hormone action: (a) EDCs inappropriately bind and activate hormone receptors, (b) EDCs block the effects of endogenous hormone by acting as receptor antagonists, (c) EDCs alter hormone receptor expression, (d) EDCs alter signal transduction in hormone-responsive cells, (e) EDCs induce epigenetic modifications in hormone-producing or hormone-responsive cells, (f) EDCs alter hormone synthesis, (g) EDCs alter hormone transport across cell membranes, (h) EDCs alter hormone distribution or circulating levels of hormones, (i) EDCs alter hormone metabolism or clearance, and (j) EDCs alter the total number or positioning of cells in hormone-producing or hormone-responsive tissues by disrupting or promoting cellular differentiation, proliferation, migration or death. It is generally agreed that an endocrine-disrupting chemical would exert its action by any one or combination of any of the aforementioned mechanisms of action (La Merrill et al., 2020).
Polychlorinated dibenzofurans (PCDFs) are a group of toxic and highly persistent aromatic organic pollutants that are generated unintentionally during incineration of chlorine-containing waste materials and as unwanted by-products in some chemical manufacturing processes (Liu et al., 2004, Lemieux et al., 2000, Minh et al., 2003, Chakraborty et al., 1243). Adverse effects of PCDF exposure on humans and wildlife have been reported in several clinical, epidemiological and laboratory animal studies. For instance, exposure to individual PCDF molecule or mixture of PCDFs in combination with other polychlorinated aromatic organic compounds has been shown to be associated with fetal anomalies in mouse (Birnbaum et al., 1987), increased proportion of spontaneous abortions and preterm deliveries among exposed women in Japan (Tsukimori et al., 2008), and growth retardation, delayed cognitive development, increased rates of hyperpigmentation, dystrophic finger-nails, acne, and swollen gum among prenatally exposed children in Taiwan (Guo et al., 1995, Guo et al., 2004, Gladen et al., 1990). Although it is generally believed that most of the toxic effects of PCDFs and other dioxin-like compounds are endocrine-related and are mediated through binding to aryl hydrocarbon receptor (AhR) (Safe, 1986, Rowlands and Gustafsson, 1997, Farrell et al., 1987, Birnbaum, 1994, Heuvel and Lucier, 1993, Beger and Wilkes, 2001, Tuomisto, 2019), review of recent literature suggests that nuclear receptors are also potential binding targets for dioxins and dioxin-like compounds (Casati et al., 2013, Portigal et al., 2002, Suzuki et al., 2011). While the mechanism involving AhR-mediated PCDF toxicity is well documented in the literature, those involving nuclear receptors have not been sufficiently investigated. Therefore, the objective of the current research was to computationally predict the susceptibility of the endocrine system to disruption by PCDFs via nuclear receptor binding.
2. Materials and methods
2.1. Hardware and software
All the computational methods described in this paper were performed using a laptop computer with the following description: HP Pavilion 15-cs0042cl, Intel® CoreTMi7-8550U processor with 16 GB DDR4 RAM, 1 TB hard drive, 2.20 Hz processor and NVIDIA® GeForce® Mx150 Graphics 4G of dedicated video and window® 10 operating system. The software used for the computational study described in this paper include: endocrine disruptome (Kolšek et al., 2014), Spartan '14 program suite (Shao et al., 2006), Molegro Virtual Docker 6.0 (Thomsen and Christensen, 2006) and Discovery Studio Visualizer (BIOVIA, Dassault Systèmes. Discovery Studio Visualizer, v20.1.0., 2019).
2.2. PCDF congeners and nuclear receptors
All the 135 possible congeners of PCDF were used as exogenous ligands in this research. The names and the structural formulae of these PCDF congeners are presented in Table S1 (Supplementary Material). Similarly, high resolution crystal structures of 12 nuclear receptors were used as protein targets. These 12 nuclear receptors are androgen receptor (PDB ID: 3L3X), estrogen receptor α (PDB ID: 1A52), estrogen receptor β (PDB ID: 3OLS), glucocorticoid receptor (PDB ID: 4P6X), liver X receptor α (PDB ID: 3IPS), liver X receptor β (PDB ID: 1P8D), peroxisome proliferator activated receptor α (PDB ID: 3KDU), peroxisome proliferator activated receptor β (PDB ID: 3GZ9), peroxisome proliferator activated receptor γ (PDB ID: 3ET3), retinoid X receptor α (PDB ID: 1MV9), thyroid hormone receptor α (PDB ID: 3ILZ) and thyroid hormone receptor β (PDB ID: 3IMY). Additionally, androgen receptor (PDB ID: 1Z95), estrogen receptor α (PDB ID: 1SJ0), estrogen receptor β (PDB ID: 1QKN) and glucocorticoid receptor (PDB ID: 3 N52) were also tested in their antagonistic conformations, bringing the total number of protein targets to 16. Detailed descriptions of the co-crystallized ligands, resolutions of the protein structures, and sources of nuclear receptors used in this computational study are presented in Table S2.
2.3. Computational screening of PCDF molecules and nuclear receptors
The probability of each of the 135 PCDF molecules displayed in Table S1 (Supplementary Material) to bind with each of the 16 protein targets listed in Table S2 (Supplementary Material) was assessed using endocrine disruptome (http://endocrinedisruptome.ki.si/). Endocrine disruptome is a computational resource designed as a user-friendly, open-source, web-based prediction tool, intended for screening large numbers of chemical substances suspected to be potential EDCs. It runs on a platform called Docking interface for Target Systems (DoTS) and it uses AutoDock Vina in the background to perform docking (Kolšek et al., 2014). Results obtained from this computational tool were colour-coded into four probability binding classes on the basis of three threshold values of sensitivity (SE). Colour red (SE < 0.25) indicates high probability of binding, colour orange (0.25 < SE < 0.50) and colour yellow (0.5 < SE < 0.75) indicate intermediate binding probability, and colour green (SE > 0.75) indicates low probability of binding (Kolšek et al., 2014). The performance of endocrine disruptome was well-validated by Kolšek et al. (Kolšek et al., 2014). It should be realized that the predictions made by endocrine disruptome are not equally reliable for the different protein targets available for testing in the software, as some targets suffer from bias (Kolšek et al., 2014). Endocrine disruptome has been successfully employed for the prediction of endocrine-disrupting potentials of some chemical substances (Akinola et al., 2021, Akinola et al., 2021, Kenda and Dolenc, 2020, Plošnik et al., 2015, Usman and Ahmad, 2019, Wang et al., 2020).
2.4. Detailed molecular docking simulation
PCDF molecules predicted to exhibit high probabilities of binding to nuclear receptors from the procedure described in section 2.3 were subject to detailed molecular docking simulation. Two-dimensional (2D) structures of the selected PCDF molecules were sketched and converted into three-dimensional (3D) structures using Spartan '14 program suite (Shao et al., 2006). The 3D structures of the molecules were then optimized using semi-empirical AM1 model in the Spartan '14 program suite. The crystal structures of the receptors that show high probabilities of binding with the PCDF molecules were retrieved from protein data bank (https://www.rcsb.org/) and molecular docking simulations were then carried out between the PCDF molecules and the receptors using Molegro Virtual Docker 6.0 (Thomsen and Christensen, 2006). Iterated Simplex was chosen as search algorithm and the poses generated were scored and ranked using MolDock scoring function. Visualization of the 2D structures of the docked ligand-receptor complexes was done using Discovery Studio Visualizer (BIOVIA, Dassault Systèmes. Discovery Studio Visualizer, v20.1.0., 2019). The procedure described above was repeated for the co-crystallized ligands in the experimentally determined crystallographic structures of the affected receptors in order to generate data for comparative analysis.
2.5. Validation of docking methodology
The performance of the docking methodology described in section 2.4 was evaluated using pose selection approach (Hevener et al., 2009). In this approach, the co-crystallized ligand found in the crystal structure of a protein target was re-docked into its native binding site. The multiple poses generated from this re-docking procedure were then ranked and the root-mean-square deviation (RMSD) between the highest-ranked pose and the reference ligand in the experimental crystal structure of the target protein was calculated. Performance of docking methodology is considered successful if the RMSD between the highest-ranked pose and the reference ligand in the experimental crystal structure of protein target is<2.0 Å (Thomsen and Christensen, 2006, Hevener et al., 2009, Kramer et al., 1999). To evaluate the performance of the docking algorithms employed in the docking program used in this paper, the crystal structures of agonistic conformations of androgen receptor, estrogen receptor α, estrogen receptor β, glucocorticoid receptor, thyroid hormone receptor α and thyroid hormone receptor β as well as the crystal structures of antagonistic conformations of androgen receptor, estrogen receptor α and estrogen receptor β were retrieved from RCSB Protein Data Bank (https://www.rcsb.org/) and the co-crystallized ligands in these crystal structures were re-docked to their native binding sites. The highest-ranked poses generated in this process were superimposed on the bound ligands in the experimental crystal structures of the selected protein targets. Only the co-crystallized ligands in the crystal structures of the protein targets that showed moderate or high probabilities of binding to PCDF molecules in the procedure described in section 2.3 were used in this validation protocol.
3. Results
The results of the computational screening procedure described in section 2.3 are shown in Table S3 (Supplementary Material) and these results are summarized as the distribution of PCDF molecules based on their probabilities of binding to nuclear receptors in Fig. 1. As shown in Fig. 1, antagonistic conformation of glucocorticoid receptor as well as agonistic conformations of liver X receptor α, liver X receptor β, peroxisome proliferator activated receptor α, peroxisome proliferator activated receptor β, peroxisome proliferator activated receptor γ and retinoid X receptor α exhibit low probabilities of binding to all the 135 PCDF congeners. In Fig. 1, agonistic conformations of androgen receptor, estrogen receptor α, estrogen receptor β, glucocorticoid receptor, thyroid hormone receptor α and thyroid hormone receptor β exhibit moderate binding probabilities with 102, 30, 46, 94, 106 and 127 PCDF congeners respectively. Fig. 1 also reveals that the number of PCDF congeners that exhibit moderate binding probabilities to androgen receptor, estrogen receptor α and estrogen receptor β in their antagonistic conformations are 115, six and two respectively. It was also shown in Fig. 1 that androgen receptor in its agonistic and antagonistic conformations exhibit high probabilities of binding to two and 20 PCDF congeners respectively. The results just presented suggest that while estrogen receptor α, estrogen receptor β, glucocorticoid receptor, thyroid hormone receptor α and thyroid hormone receptor β may likely bind to some PCDF congeners, there is a strong indication that androgen receptor is highly susceptible to binding by some PCDF congeners.
Fig. 1.
Distribution of PCDF molecules based on their probabilities of binding to nuclear receptors as obtained from endocrine disruptome software.
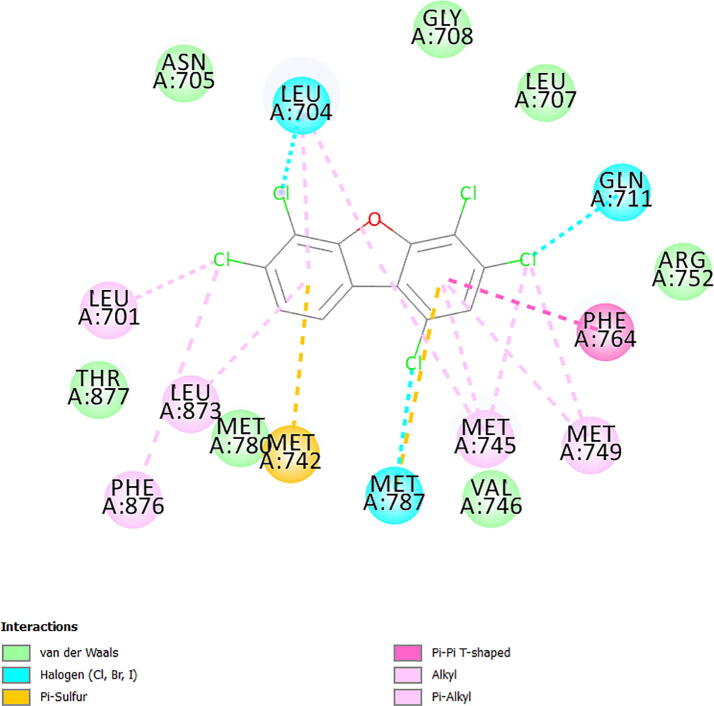
Focusing on androgen receptor due to its high probabilities of binding to PCDF congeners, the outputs of the results obtained for detailed molecular docking simulation of the two PCDF congeners that showed high probabilities of binding to agonistic conformation of androgen receptor are displayed as 2D visualizations in Figs. S1 and S2 (Supplementary Material). Similarly, the outputs of the results obtained for detailed molecular docking simulation of the 20 PCDF congeners that showed high probabilities of binding to antagonistic conformation of androgen receptor are displayed as 2D visualizations in Figs. S3 to S22 (Supplementary Material). Due to limitation imposed by space, the ligand-receptor complexes displayed in Figs. S1 to S22 are exemplified with the output of the result obtained for the binding interaction of 2,3,4,6,7-pentachlorodibenzofuran (PCDF 112) with amino acid residues in the binding pocket of agonistic conformations of androgen receptor (Fig. S2 in the Supplementary Material) and the output of the result obtained for the binding interaction of 1,3,4,6,7-pentachlorodibenzofuran (PCDF 104) with amino acid residues in the binding pocket of antagonistic conformations of androgen receptor (Fig. S16 in the Supplementary Material). Comparing the amino acid residues in the binding pocket of agonistic conformation of androgen receptor that formed binding interactions with testosterone (Fig. 2) and PCDF 112 (Fig. 3) and the amino acid residues in the binding pocket of antagonistic conformation of androgen receptor that formed binding interactions with bicalutamide (Fig. 4) and PCDF 104 (Fig. 5) revealed that both PCDF 112 and PCDF 104 formed ligand-receptor complexes that were stabilized by electrostatic, van der Waals, pi-effect and hydrophobic interactions, mimicking the modes of interaction observed in androgen-testosterone complex (Fig. 2) and androgen-bicalutamide complex (Fig. 4) respectively. The essential features of these results are summarized in Table 1. Analysis of the docking results presented in Table 1 indicates that both testosterone and 2,3,4,6,7-pentachlorodibenzofuran formed non-covalent interactions with 21 and 17 amino acid residues in the active site of agonistic conformation of androgen receptor, utilizing Arg-752, Asn-705, Gln-711, Leu-701, Leu-704, Leu-707, Leu-873, Leu-880, Met-742, Met-745, Met-749, Met-780, Met-787, Phe-764, Phe-876, Thr-877, Val-746 as common amino acid residues. Table 1 also indicates that both bicalutamide and 1,3,4,6,7-pentachlorodibenzofuran formed non-covalent interactions with 23 and 17 amino acid residues in the active site of antagonistic conformation of androgen receptor, utilizing Arg-752, Asn-705, Gln-711, Gly-708, Leu-701, Leu-704, Leu-707, Leu-873, Met-742, Met-745, Met-749, Met-780, Met-787, Phe-764, Phe-876, Thr-877, Val-746 as common amino acid residues. The amino acid residues utilized by 2,3,4,6,7-pentachlorodibenzofuran and 1,3,4,6,7-pentachlorodibenzofuran for binding correspond to about 81% and 74% of the amino acid residues used by testosterone and bicalutamide for binding with androgen receptor in its agonistic and antagonistic conformations respectively. The high degree of commonalities of the interacting amino acid residues that stabilized the receptor-ligand complexes formed by PCDF molecules and the co-crystallized ligands in the crystal structures of androgen receptor suggests the possibility of some PCDF congeners acting as endocrine disruptors by mimicking and antagonizing the binding actions of testosterone. Values of MolDock scores presented in Table 1 for the binding of 2,3,4,6,7-pentachlorodibenzofuran (-92.75 kcal/mol) and 1,3,4,6,7-pentachlorodibenzofuran (-95.47 kcal/mol) with androgen receptor are lower than the MolDock scores obtained for the binding of testosterone (-111.37 kcal/mol) and bicalutamide (-142.05 kcal/mol) with androgen receptor. This indicates that PCDF molecules bind to androgen receptor with lower binding affinities than testosterone and bicalutamide.
Fig. 2.
Amino acid residues in the binding pocket of agonistic conformation of androgen receptor (PDB ID: 3L3X) involved in non-covalent interactions with testosterone.
Fig. 3.
Amino acid residues in the binding pocket of agonistic conformation of androgen receptor (PDB ID: 3L3X) involved in non-covalent interactions with 2,3,4,6,7-pentachlorodibenzofuran (PCDF 112).
Fig. 4.
Amino acid residues in the binding pocket of antagonistic conformation of androgen receptor (PDB ID: 1Z95) involved in non-covalent interactions with bicalutamide.
Fig. 5.
Amino acid residues in the binding pocket of antagonistic conformation of androgen receptor (PDB ID: 1Z95) involved in non-covalent interactions with 1,3,4,6,7-pentachlorodibenzofuran (PCDF 104).
Table 1.
MolDock scores and common amino acid residues involved in ligand-receptor binding interactions.
| Receptor | Ligand | MolDock score (kcal/mol) | Amino acid residues involved in binding interactions | Common amino acid residues involved in binding interactions |
|---|---|---|---|---|
| AR | Testosterone | −111.37 | Arg-752, Asn-705, Gln-711, Gly-708, Leu-701, Leu-704, Leu-707, Leu-873, Leu-880, Met-742, Met-745, Met-749, Met-780, Met-787, Met-895, Phe-764, Phe-876, Phe-891, Thr-877, Trp-741, Val-746 | Arg-752, Asn-705, Gln-711, Leu-701, Leu-704, Leu-707, Leu-873, Leu-880, Met-742, Met-745, Met-749, Met-780, Met-787, Phe-764, Phe-876, Thr-877, Val-746 |
| PCDF 112 | −92.72 | Arg-752, Asn-705, Gln-711, Leu-701, Leu-704, Leu-707, Leu-873, Leu-880, Met-742, Met-745, Met-749, Met-780, Met-787, Phe-764, Phe-876, Thr-877, Val-746 | ||
| AR an | Bicalutamide | −142.05 | Arg-752, Asn-705, Gln-711, Gly-708, Ile-899, Leu-701, Leu-704, Leu-707, Leu-768, Leu-873, Leu-880, Met-742, Met-745, Met-749, Met-780, Met-787, Met-895, Phe-764, Phe-876, Phe-891, Thr-877, Trp-741, Val-746 | Arg-752, Asn-705, Gln-711, Gly-708, Leu-701, Leu-704, Leu-707, Leu-873, Met-742, Met-745, Met-749, Met-780, Met-787, Phe-764, Phe-876, Thr-877, Val-746 |
| PCDF 104 | −95.47 | Arg-752, Asn-705, Gln-711, Gly-708, Leu-701, Leu-704, Leu-707, Leu-873, Met-742, Met-745, Met-749, Met-780, Met-787, Phe-764, Phe-876, Thr-877, Val-746 |
The results obtained for the procedure described in Section 2.5 for evaluating the performance of the docking protocol employed in this paper are presented in Fig. S23 (Supplementary Material). As shown in Fig. S23, visual inspection of the theoretically-generated poses and the bound ligands in the experimental crystal structures of the selected protein targets indicates that all the poses aligned well with the experimental reference ligands. Fig. S23 also shows that the values of the root-mean-square deviation (RMSD) ranged from 0.392 Å for structure with PDB code 1Z95 to 0.978 Å for structure with PDB code 1SJ0. Since the values of the RMSD shown in Fig. S23 are less than the threshold value of 2.0 Å, the docking protocol used in Molegro Virtual Docker 6.0 is adjudged to be accurate for the current computational research.
4. Discussion
The susceptibility of twelve nuclear receptors to binding by PCDFs was investigated using molecular docking simulation. The computational results obtained suggest that some PCDF congeners may act as agonists and antagonists of androgen receptor, thereby causing endocrine disruption. Although the use of experimental techniques for toxicity assessment is considered more reliable than computational techniques, utility of experiments for large-scale screening of potential endocrine-disrupting chemicals are known to be more expensive, time-consuming and low-throughput (Lang et al., 2018, Sakkiah et al., 2019, Shukla et al., 2010, Tukker et al., 2016). Androgen receptor is a ligand-activated transcription factor and a member of the steroid hormone receptor family that binds with high affinity to testosterone and other androgens (Davey and Grossmann, 2016, Wilson et al., 2003). Binding of testosterone to androgen receptor is known to be responsible for maturation of male reproductive organs, development of male secondary sexual characteristics at puberty, and muscular development in men (Marieb and Hoehn, 2019, Dohle et al., 2003, Wyce et al., 2010). It is well documented in literature that exposure of humans and animals to complex mixtures of PCDFs and other structurally-related polychlorinated aromatic organic compounds is associated with increased abnormal sperm morphology, reduced sperm motility, reduced capacity of sperm to penetrate hamster oocytes, and increased risk of prostate cancer (Guo et al., 2000, Vested et al., 2014, 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure and prostate cancer: a meta-analysis of cohort studies. Public Health., 2014). To the best of our knowledge, no experimental report of PCDF binding to androgen receptor is available in the literature. Activation of signal transduction or blocking of hormonal action of testosterone, initiated by binding of PCDFs to androgen receptor as predicted theoretically in this paper, may perhaps provide plausible explanation for some of the harmful effects of PCDFs on male reproductive health.
The computational results presented in this paper also indicate that PCDFs bind to androgen receptor with binding affinities that are lower than the binding affinities of testosterone and bicalutamide. This finding is consistent with previously reported results for other low-molecular weight exogenous chemicals that act as endocrine disruptors—they generally act as weak agonists or antagonists with binding affinities several orders of magnitude lower than the binding affinities of endogenous hormones (Autrup et al., 2020, Balaguer et al., 2017, Shanle and Xu, 2011, Lee et al., 2013). The relatively lower binding affinities of PCDFs and other low-molecular weight EDCs may be due to the fewer contacts that these EDCs made within the binding cavities of the receptors on account of their small sizes (Balaguer et al., 2017). In contrast, endogenous ligands could form more effective interactions within the binding cavities of the receptors because of their relatively larger sizes (Balaguer et al., 2017). It should be noted however that, despite the lower binding affinities of PCDFs, undesirable toxic effects might still occur through additive effects of PCDFs and other exogenous chemicals since humans and wildlife are usually exposed to multiple endocrine disruptors in their diets and environment, and these multiple endocrine disruptors may be acting through a common mechanism, such as through binding to a specific biological target (Darbre and Darbre, 2015). Evaluating the performance of docking protocol employed in this paper resulted in RMSD values that are lower than the threshold value of 2.0 Å for all the tested receptor-ligand complexes. The high level of accuracy obtained in this paper is in agreement with what has been reported in the literature on the performance of MolDock scoring algorithm (Thomsen and Christensen, 2006). The docking protocol used in the current research is therefore considered accurate and reliable.
5. Conclusion
Many exogenous chemicals found in diets, environment, industrial products and personal care products can cause endocrine disruption via ten identifiable key mechanisms according to consensus of opinion among experts. Parts of these mechanisms involve binding of exogenous chemicals to nuclear receptors, leading to inappropriate activation of signal transduction or blocking the actions of endogenous hormones by acting as receptor antagonists. Using molecular docking simulation, it was demonstrated in this paper that some PCDF congeners may cause endocrine disruption in humans and wildlife by inappropriately binding to androgen receptor in its agonistic and antagonistic conformations. Although the findings obtained in this paper may provide plausible explanation for the widely reported male reproductive dysfunctions observed in humans and wildlife due to PCDF exposure, the high number of positives predicted for androgen receptor antagonism by endocrine disruptome should be interpreted with caution since this software suffers from a high false positive rate for androgen receptor antagonism. In view of this caveat, the prediction made in the current computational study should be viewed as tentative and further experimental investigation specifically designed to confirm or refute the theoretical findings reported in this paper is suggested.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank Dr. David Ebuka Arthur for providing the molegro virtual docker 6.0 software used in this research work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2021.09.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Caliman F.A., Gavrilescu M. Pharmaceuticals, personal care products and endocrine disrupting agents in the environment- a review. Clean-Soil, Air, Water. 2009;37(4-5):277–303. [Google Scholar]
- Zhang Z., Jia C., Hu Y., Sun L., Jiao J., Zhao L., Zhu D., Li J., Tian Y., Bai H., Li R., Hu J. The estrogenic potential of salicylate esters and their possible risks in foods and cosmetics. Toxicol. Lett. 2012;209(2):146–153. doi: 10.1016/j.toxlet.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Muncke J. Exposure of endocrine disrupting compounds via the food chain: is packaging a relevant source? Sci. Total Environ. 2009;407:4549–4559. doi: 10.1016/j.scitotenv.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Koo H.J., Lee B.M. Estimated exposure to phthalates in cosmetics and risk assessment. J. Toxicol. Environ. Health, Part A. 2004;67(23-24):1901–1914. doi: 10.1080/15287390490513300. [DOI] [PubMed] [Google Scholar]
- Rudel R.A., Camann D.E., Spengler J.D. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003;37:4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Darbre P.D. Overview of air pollution and endocrine disorders. Int. J. General Med. 2018;11:191–207. doi: 10.2147/IJGM.S102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pojana G., Gomiero A., Jonkers N., Marcomini A. Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environ. Int. 2007;33(7):929–936. doi: 10.1016/j.envint.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rhind S.M., Kyle C.E., Ruffie H., Calmettes E., Osprey M., Zhang Z.L., Hamilton D., McKenzie C. Short- and long-term temporal changes in soil concentrations of selected endocrine disrupting compounds (EDCs) following single or multiple applications of sewage sludge to pastures. Environ. Pollut. 2013;181:262–270. doi: 10.1016/j.envpol.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Archer E., Wolfaardt G.M., van Wyk J.H. Pharmaceutical and personal care products (PPCPs) as endocrine disrupting contaminants (EDCs) in South African surface waters. Water SA. 2017;43:684–706. doi: 10.1016/j.chemosphere.2017.01.101. [DOI] [PubMed] [Google Scholar]
- Loffredo E., Senesi N. Fate of anthropogenic organic pollutants in soils with emphasis on adsorption/desorption processes of endocrine disruptor compounds. Pure Appl. Chem. 2006;78(5):947–961. [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J.P., Giudice L.C. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre P.D. In: Endocrine Disruption and Human Health. Darbre P.D., editor. Elsevier; Amsterdam (NL): 2015. How could endocrine disrupters affect human health? pp. 27–45. [Google Scholar]
- Gore A.C., Chappell V.A., Fenton S.E. EDC2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre P.D. In: Endocrine Disruption and Human Health. Darbre P.D., editor. Elsevier; Amsterdam (NL): 2015. What are endocrine disrupters and where are they found? pp. 3–26. [Google Scholar]
- Miodovnik A., Engel S.M., Zhu C., Ye X., Soorya L.V., Silva M.J., Calafat A.M., Wolff M.S. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug T.T., Janesick A., Blumberg B., Heindel J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011;127(3-5):204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson A., Bourguignon J.P., Parent A.S. Exposure to endocrine disrupting chemicals and neurodevelopmental alterations. Andrology. 2016;4:706–722. doi: 10.1111/andr.12211. [DOI] [PubMed] [Google Scholar]
- Rogers J.A., Metz L., Yong V.W. Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013;53(4):421–430. doi: 10.1016/j.molimm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Wang C., Yang L., Wang S. The classic EDCs, phthalate esters and oranochlorines, in relation to abnormal sperm quality: a systematic review with meta-analysis. Scientific Report. 2016;6:1–11. doi: 10.1038/srep19982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamkowska D., Karwacka A., Jurewicz J. Environmental exposure to non-persistent endocrine disrupting chemicals and semen quality: an overview of the current epidemiological evidence. Int. J. Occup. Med. Environ. Health. 2018;31(4):377–414. doi: 10.13075/ijomeh.1896.01195. [DOI] [PubMed] [Google Scholar]
- Hu W.Y., Shi G.B., Hu D.P., Nelles J.L., Prins G.S. Actions of estrogens and endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Mol. Cell. Endocrinol. 2012;354(1-2):63–73. doi: 10.1016/j.mce.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess-Wilson J.K., Knudsen K.E. Endocrine disrupting compounds and prostate cancer. Cancer Lett. 2006;241(1):1–12. doi: 10.1016/j.canlet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Soto A.M., Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010;6(7):363–370. doi: 10.1038/nrendo.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M.F., Olea N. Endocrine Disruptors and Puberty. Humana Press; Totowa, NJ: 2012. pp. 225–239. [DOI] [Google Scholar]
- Yum T., Lee S., Kim Y. Association between precocius puberty and some endocrine disruptors in human plasma. J. Environ. Sci. Health, Part A. 2013;48:912–917. doi: 10.1080/10934529.2013.762734. [DOI] [PubMed] [Google Scholar]
- Grindler N.M., Allsworth J.E., Macones G.A. Persistent organic pollutants and early menopause in U.S. women. PLoS ONE. 2015;10:1–12. doi: 10.1371/journal.pone.0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. In: Estrogen Receptor and Breast Cancer: Celebrating the 60th Anniversary of the Discovery of ER. Zhang X., editor. Humana Press; Totowa (NJ): 2019. Estrogen receptor and breast cancer: a historical perspective; pp. 1–14. [Google Scholar]
- Alonso-Magdalena P., Quesada I., Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011;7(6):346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- Newbold R.R., Padilla-Banks E., Jefferson W.N., Heindel J.J. Effects of endocrine disruptors on obesity. Int. J. Androl. 2008;31(2):201–208. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- Hatch E.E., Nelson J.W., Stahlhut R.W. Association of endocrine disruptors and obesity: perspectives from epidemiological studies. Int. J. Androl. 2010;33:324–332. doi: 10.1111/j.1365-2605.2009.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige J.N., Gelman L., Rossi D., Zoete V., Métivier R., Tudor C., Anghel S.I., Grosdidier A., Lathion C., Engelborghs Y., Michielin O., Wahli W., Desvergne B. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor γ modulator that promotes adipogenesis. J. Biol. Chem. 2007;282(26):19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- Hao C., Cheng X., Xia H. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci. Rep. 2012;32:619–629. doi: 10.1042/BSR20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Molina J.-M., Amaya E., Grimaldi M., Sáenz J.-M., Real M., Fernández M.F., Balaguer P., Olea N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013;272(1):127–136. doi: 10.1016/j.taap.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Rubin B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011;127(1-2):27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Matsushima A., Kakuta Y., Teramoto T., Koshiba T., Liu X., Okada H., Tokunaga T., Kawabata S.-i., Kimura M., Shimohigashi Y. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERRγ. J. Biochem. 2007;142(4):517–524. doi: 10.1093/jb/mvm158. [DOI] [PubMed] [Google Scholar]
- Vitku J., Kolatorova L., Franekova L. Endocrine disruptors of the bisphenol and paraben families and bone metabolism. Physiol. Res. 2018;67(3):S455–S464. doi: 10.33549/physiolres.934005. [DOI] [PubMed] [Google Scholar]
- Kolatorova L., Sramkova M., Vitku J. Parabens and their relation to obesity. Physiol. Res. 2018;67(3):S465–S472. doi: 10.33549/physiolres.934004. [DOI] [PubMed] [Google Scholar]
- Raut S.A., Angus R.A. Triclosan has endocrine-disrupting effects in male western mosquitofish, Gambusia affinis. Environ. Toxicol. Chem.. 2010;29:1287–1291. doi: 10.1002/etc.150. [DOI] [PubMed] [Google Scholar]
- Foran C.M., Bennett E.R., Benson W.H. Developmental evaluation of a potential non-steroidal estrogen: triclosan. Marine Environmental Research. 2000;50(1-5):153–156. doi: 10.1016/s0141-1136(00)00080-5. [DOI] [PubMed] [Google Scholar]
- Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters—what we learned from Yusho disease. Environ. Res. 2001;86:2–11. doi: 10.1006/enrs.2001.4244. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Miyazaki W., Takeshita A., Kuroda Y., Koibuchi N. Polychlorinated biphenyls suppress thyroid hormone-induced transactivation. Biochemical and Biophysical Research Communication. 2002;299(3):384–388. doi: 10.1016/s0006-291x(02)02659-1. [DOI] [PubMed] [Google Scholar]
- Goldey E.S., Kehn L.S., Lau C. Developmental exposure of polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol. Appl. Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Fernandez M., Paradisi M., D’Intino G. A single prenatal exposure to the endocrine disruptor 2,3,7,8–tetrachlorodibenzo-p-dioxin alters developmental myelination and remyelination potential in the rat brain. J. Neurochem. 2010;115:897–909. doi: 10.1111/j.1471-4159.2010.06974.x. [DOI] [PubMed] [Google Scholar]
- Ünüvar T., Büyükgebiz A. Fetal and neonatal endocrine disruptors. J. Clin. Res. Pediatr. Endocrinol. 2012;4(2):51–60. doi: 10.4274/Jcrpe.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Gao Y., Guo L.H. Structure-dependent activities of hydroxylated polyrominated diphenyl ethers on human estrogen receptor. Toxicology. 2013;309:15–22. doi: 10.1016/j.tox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Yue S., Zhang T., Shen Q., Song Q., Ji C., Chen Y., Mao M., Kong Y., Chen D.a., Liu J., Sun Z., Zhao M. Assessment of endocrine-disrupting effects of emerging polyhalogenated carbazoles (PHCZs): in vitro, in silico, and in vivo evidence. Environ. Int. 2020;140:105729. doi: 10.1016/j.envint.2020.105729. [DOI] [PubMed] [Google Scholar]
- Santti R., Mäkelä S., Strauss L., Korkman J., Kostian M.-L. Phytoestrogens: potential endocrine disruptors in males. Toxicol. Ind. Health. 1998;14(1-2):223–237. doi: 10.1177/074823379801400114. [DOI] [PubMed] [Google Scholar]
- Zielonka Ł., Waśkiewicz A., Beszterda M., Kostecki M., Dąbrowski M., Obremski K., Goliński P., Gajęcki M. Zearalenone in the intestinal tissues of immature gilts exposed per os to mycotoxins. Toxins. 2015;7(8):3210–3223. doi: 10.3390/toxins7083210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejdovszky K., Hahn K., Braun D., Warth B., Marko D. Synergistic estrogenic effects of Fusarium and Alternaria mycotoxins in vitro. Arch. Toxicol. 2017;91(3):1447–1460. doi: 10.1007/s00204-016-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El. Khoury D., Fayjaloun S., Nassar M., Sahakian J., Aad P.Y. Updates on the effect of mycotoxins on male reproductive efficiency in mammals. Toxins. 2019;11(9):515. doi: 10.3390/toxins11090515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combarnous Y. Endocrine disruptor compounds (EDCs) and agriculture: the case of pesticides. C.R. Biol. 2017;340(9-10):406–409. doi: 10.1016/j.crvi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Leemans M., Couderq S., Demeneix B. Pesicides with potential thyroid hormone-disrupting effects: a review of recent data. Front. Endocrinol. 2019;10:743. doi: 10.3389/fendo.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M.A., Vandenberg L.N., Smith M.T., Goodson W., Browne P., Patisaul H.B., Guyton K.Z., Kortenkamp A., Cogliano V.J., Woodruff T.J., Rieswijk L., Sone H., Korach K.S., Gore A.C., Zeise L., Zoeller R.T. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020;16(1):45–57. doi: 10.1038/s41574-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Zheng M., Wang D. Formation of PCDD/Fs PCBs in the process of production of 1,4-dichlorobenzene. Chemosphere. 2004;57:1317–1323. doi: 10.1016/j.chemosphere.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Lemieux P.M., Lutes C.C., Abbott J.A., Aldous K.M. Emissions of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans from the open burning of household waste in barrels. Environ. Sci. Technol. 2000;34(3):377–384. [Google Scholar]
- Minh N.H., Minh T.B., Watanabe M. Open dumping site in Asian developing countries: a potential source of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. Environ. Sci. Technol. 2003;37:1493–1502. doi: 10.1021/es026078s. [DOI] [PubMed] [Google Scholar]
- P. Chakraborty, S. Selvaraj, M. Nakamura, et al. E-waste and associated environmental contamination in the Asia/Pacific region (Part 2): a case study of dioxins and furans in e-waste recycling/dump sites in India. In: Loganathan BG, Khim JS, Kodavanti PRS, et al., editiors. Persistent Organic Chemicals in the Environment: Status and Trends in the Pacific Basin Countries. ACS symposium series 1243. Washinton, D.C. (WA): American Chemical Society; 2016. p. 139–154.
- Birnbaum L.S., Harris M.W., Crawford D.D. Teratogenic effects of polychlorinated dibenzofurans in combination in C57BL/6N mice. Toxicol. Appl. Pharmacol. 1987;91:246–255. doi: 10.1016/0041-008x(87)90105-0. [DOI] [PubMed] [Google Scholar]
- Tsukimori K., Tokunaga S., Shibata S. Long-term effects of polychlorinated biphenyls and dioxins on pregnancy outcomes in women affected by Yusho incident. Environ. Health Perspect. 2008;116(5):626–630. doi: 10.1289/ehp.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.L., Lambert G.H., Hsu C.C. Growth abnormalities in the population exposed in utero and early postnatally to polychlorinated biphenyls and dibenzofurans. Environ. Health Perspect. 1995;103(6):117–122. doi: 10.1289/ehp.95103s6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.L., Lambert G.H., Hsu C.-C., Hsu M.M.L. Yucheng: health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int. Arch. Occup. Environ. Health. 2004;77(3):153–158. doi: 10.1007/s00420-003-0487-9. [DOI] [PubMed] [Google Scholar]
- Gladen B.C., Taylor J.S., Wu Y.C. Dermatological findings in children exposed transplacentally to heat-degraded polychlorinated biphenyls in Taiwan. Br. J. Dermatol. 1990;122(6):799–808. doi: 10.1111/j.1365-2133.1990.tb06269.x. [DOI] [PubMed] [Google Scholar]
- Safe S.H. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu. Rev. Pharmacol. Toxicol. 1986;26(1):371–399. doi: 10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- Rowlands J.C., Gustafsson J.-Å. Aryl hydrocarbon receptor-mediated signal transduction. Crit. Rev. Toxicol. 1997;27(2):109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- Farrell K., Safe L., Safe S. Synthesis and aryl hydrocarbon receptor binding properties of radiolabeled polychlorinated dibenzofuran congeners. Arch. Biochem. Biophys. 1987;259(1):185–195. doi: 10.1016/0003-9861(87)90485-1. [DOI] [PubMed] [Google Scholar]
- Birnbaum L.S. The mechanism of dioxin toxicity: relationship to risk assessment. Environ. Health Perspect. 1994;102(suppl 9):157–167. doi: 10.1289/ehp.94102s9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuvel J.P.V., Lucier G. Environmental toxicology of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. Environ. Health Perspect. 1993;100:189–200. doi: 10.1289/ehp.93100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger R.D., Wilkes J.G. Models of polychlorinated dibenzodioxins, dibbenzofurans, and biphenyls binding affinity to aryl hydrocarbon receptor developed using 13C NMR data. J. Chem. Inf. Comput. Sci. 2001;41:1322–1329. doi: 10.1021/ci000312l. [DOI] [PubMed] [Google Scholar]
- Tuomisto J. Dioxins and dioxin-like compounds: toxicity in humans and animals, sources, and behaviour in the environment. Wiki J. Med. 2019;6(1):8. doi: 10.15347/WJM10.15347/wjm/2019.008. [DOI] [Google Scholar]
- Casati L., Sendra R., Poletti A., Negri-Cesi P., Celotti F. Androgen receptor activation by polychlorinated biphenyls: epigenetic effects mediatiated by the histone demethylase Jarid1b. Epigenetics. 2013;8(10):1061–1068. doi: 10.4161/epi.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portigal C.L., Cowell S.P., Fedoruk M.N., Butler C.M., Rennie P.S., Nelson C.C. Polychlorinated biphenyls interfere with androgen-induced transcriptional activation and hormone binding. Toxicol. Appl. Pharmacol. 2002;179(3):185–194. doi: 10.1006/taap.2002.9371. [DOI] [PubMed] [Google Scholar]
- Suzuki G.o., Tue N.M., van der Linden S., Brouwer A., van der Burg B., van Velzen M., Lamoree M., Someya M., Takahashi S., Isobe T., Tajima Y., Yamada T.K., Takigami H., Tanabe S. Identification of major dioxin-like compounds and androgen receptor antagonist in acid-treated tissue extracts of high trophic-level animals. Environ. Sci. Technol. 2011;45(23):10203–10211. doi: 10.1021/es2024274. [DOI] [PubMed] [Google Scholar]
- Kolšek K., Mavri J., Sollner Dolenc M., Gobec S., Turk S. Endocrine Disruptome— an open source prediction tool for assessing endocrine disruption potential through nuclear receptor binding. J. Chem. Inf. Model. 2014;54(4):1254–1267. doi: 10.1021/ci400649p. [DOI] [PubMed] [Google Scholar]
- Shao Y., Molnar L.F., Jung Y., Kussmann J., Ochsenfeld C., Brown S.T., Gilbert A.T.B., Slipchenko L.V., Levchenko S.V., O’Neill D.P., DiStasio Jr R.A., Lochan R.C., Wang T., Beran G.J.O., Besley N.A., Herbert J.M., Yeh Lin C., Van Voorhis T., Hung Chien S., Sodt A., Steele R.P., Rassolov V.A., Maslen P.E., Korambath P.P., Adamson R.D., Austin B., Baker J., Byrd E.F.C., Dachsel H., Doerksen R.J., Dreuw A., Dunietz B.D., Dutoi A.D., Furlani T.R., Gwaltney S.R., Heyden A., Hirata S.o., Hsu C.-P., Kedziora G., Khalliulin R.Z., Klunzinger P., Lee A.M., Lee M.S., Liang W., Lotan I., Nair N., Peters B., Proynov E.I., Pieniazek P.A., Min Rhee Y., Ritchie J., Rosta E., David Sherrill C., Simmonett A.C., Subotnik J.E., Lee Woodcock III H., Zhang W., Bell A.T., Chakraborty A.K., Chipman D.M., Keil F.J., Warshel A., Hehre W.J., Schaefer III H.F., Kong J., Krylov A.I., Gill P.M.W., Head-Gordon M. Advances in methods and algorithms in a modern quantum chemistry program package. PCCP. 2006;8(27):3172–3191. doi: 10.1039/b517914a. [DOI] [PubMed] [Google Scholar]
- Thomsen R., Christensen M.H. MolDock: a new technique for high-accuracy molecular docking. J. Med. Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- BIOVIA, Dassault Systèmes. Discovery Studio Visualizer, v20.1.0.19295, San Diego: Dassault Systèmes; 2019.
- Akinola L.K., Uzairu A., Shallangwa G.A., Abechi S.E. Theoretical study on endocrine disrupting effects of polychlorinated dibenzo-p-dioxins using molecular docking simulation. J. Appl. Toxicol. 2021;41(2):233–246. doi: 10.1002/jat.4039. [DOI] [PubMed] [Google Scholar]
- Akinola L.K., Uzairu A., Shallangwa G.A., Abechi S.E. A computational insight into endocrine disruption by polychlorinated biphenyls via non-covalent interactions with human nuclear receptors. Ecotoxicol. Environ. Saf. 2021;214:112086. doi: 10.1016/j.ecoenv.2021.112086. [DOI] [PubMed] [Google Scholar]
- Kenda M., Dolenc M.S. Computational study of drug targeting nuclear receptors. Molecules. 2020;25(7):1616. doi: 10.3390/molecules25071616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plošnik A., Vracko M., Mavri J. Computational study of affinity to nuclear receptors for cosmetic ingredients. Chemosphere. 2015;135:325–334. doi: 10.1016/j.chemosphere.2015.04.075. [DOI] [PubMed] [Google Scholar]
- Usman A., Ahmad M. Computational study suggesting reconsideration of BPA analogues based on their endocrine disrupting potential estimated by binding affinities to nuclear receptors. Ecotoxicol. Environ. Saf. 2019;171:154–161. doi: 10.1016/j.ecoenv.2018.12.071. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang R., Song C., Crump D. Computational evaluation of interactions between organophosphate esters and nuclear hormone receptors. Environ. Res. 2020;182:108982. doi: 10.1016/j.envres.2019.108982. [DOI] [PubMed] [Google Scholar]
- Hevener K.E., Zhao W., Ball D.M., Babaoglu K., Qi J., White S.W., Lee R.E. Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J. Chem. Inf. Model. 2009;49(2):444–460. doi: 10.1021/ci800293n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B., Rarey M., Lengauer T. Evaluation of the FLEXX incremental construction algorithm for protein-ligand docking. Proteins. 1999;37(2):228–241. doi: 10.1002/(sici)1097-0134(19991101)37:2<228::aid-prot8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Lang A., Volkamer A., Behm L. In silico methods—computational alternative to animal testing. ALTEX—Alternatives to Animal Experimentation. 2018;35(1):126–128. doi: 10.14573/altex.1712031. [DOI] [PubMed] [Google Scholar]
- Sakkiah S., Kusko R., Tong W. In: Advances in Computational Toxicology: Methodologies and Applications in Regulatory Science. Hong H., editor. Springer Nature; Switzerland (CH): 2019. Applications of molecular dynamics simulations in computational toxicology; pp. 181–212. [Google Scholar]
- Shukla S.J., Huang R., Austin C.P., Xia M. The future of toxicity testing: a focus on in vitro methods using a quantitative high-throughput screening platform. Drug Discovery Today. 2010;15(23-24):997–1007. doi: 10.1016/j.drudis.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukker A.M., de Groot M.W.G.D.M., Wijnolts F.M.J. Is the time right for in vitro neurotoxicity testing using human iPSC-derived neurons? ALTEX—Alternatives to Animal Experimentation. 2016;33(3):261–271. doi: 10.14573/altex.1510091. [DOI] [PubMed] [Google Scholar]
- Davey R.A., Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clinical Biochemist Reviews. 2016;37:3–15. [PMC free article] [PubMed] [Google Scholar]
- Wilson E.M. In: Encyclopedia of Hormones. Henry H.L., Norman A.W., editors. Academic Press; London (GB): 2003. Androgen receptor structure and function; pp. 103–110. [Google Scholar]
- Marieb E.N., Hoehn K. Pearson Education, Inc.; London (GB): 2019. Human Anatomy and Physiology; pp. 601–641. [Google Scholar]
- Dohle G.R., Smit M., Weber R.F.A. Androgens and male fertility. World J. Urol. 2003;21(5):341–345. doi: 10.1007/s00345-003-0365-9. [DOI] [PubMed] [Google Scholar]
- Wyce A., Bai Y., Nagpal S. The androgen receptor modulates expression of genes with critical roles in muscle development and function. Mol. Endocrinol. 2010;24:1665–1674. doi: 10.1210/me.2010-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.L., Hsu P.-C., Hsu C.-C., Lambert G.H. Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans. The Lancet. 2000;356(9237):1240–1241. doi: 10.1016/S0140-6736(00)02792-6. [DOI] [PubMed] [Google Scholar]
- Vested A., Giwercman A., Bonde J., Toft G. Persistent organic pollutants and male reproductive health. Asian Journal of Andrology. 2014;16(1):71. doi: 10.4103/1008-682X.122345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure and prostate cancer: a meta-analysis of cohort studies. Public Health. 2014;128:207–213. [DOI] [PubMed]
- Autrup H., Barile F.A., Berry S.C. Human exposure to synthetic endocrine disrupting chemicals (S-EDCs) is generally negligible as compared to natural compounds with higher or comparable endocrine activity. How to evaluate the risk of the S-EDCs. J. Toxicol. Environ. Health, Part A. 2020;83:484–494. doi: 10.1080/15287394.2020.1756592. [DOI] [PubMed] [Google Scholar]
- Balaguer P., Delfosse V., Grimaldi M., Bourguet W. Structural and functional evidences for the interactions between nuclear hormone receptors and endocrine disruptors at low doses. C.R. Biol. 2017;340(9-10):414–420. doi: 10.1016/j.crvi.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Shanle E.K., Xu W. Endocrine disrupting chemicals targeting oestrogen receptor signalling: identification and mechanisms of actions. Chem. Res. Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-R., Jeung E.-B., Cho M.-H., Kim T.-H., Leung P.C.K., Choi K.-C. Molecular mechanism(s) of endocrine-disrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors. J. Cell Mol. Med. 2013;17(1):1–11. doi: 10.1111/j.1582-4934.2012.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.