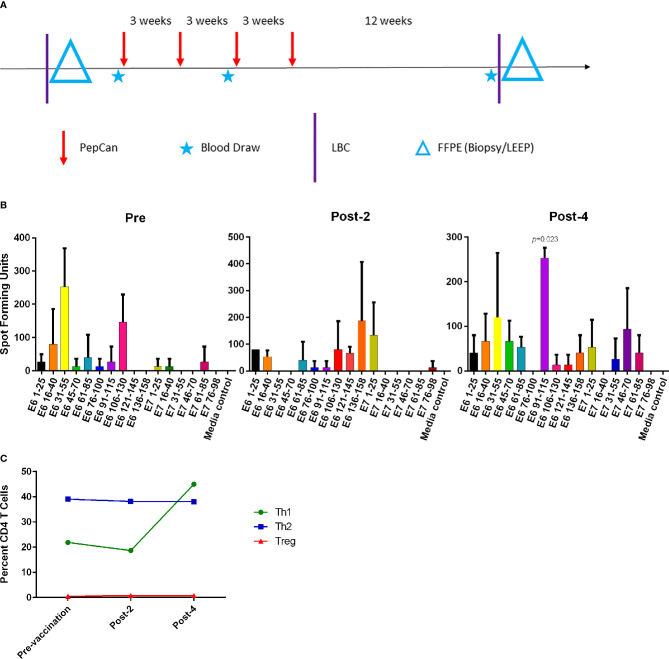
Figure 1.
The Phase I clinical trial design and routine immune monitoring assays. (A) Clinical trial design of the Phase I study. Vaccination (PepCan) visits were scheduled 3 weeks apart for patients who had biopsy-confirmed cervical high-grade squamous intraepithelial lesions (HSILs, i.e. CIN grade 2 or 3). Blood draws were performed pre-vaccination, and post-2 and post-4 vaccinations. Cervical local samples (LBC and FFPE) were collected pre-vaccination and post-4 vaccinations. FFPE samples were prepared from a pre-vaccination cervical biopsy and from loop electrical excision procedure (LEEP) biopsy post-4 vaccinations. (B) Immunogenic HPV16 E6 and E7 regions were determined for each vaccine phase using IFN-γ ELISPOT assay. In pre-vaccine phase, positive responses (i.e., at least twice the media control) were detected in the E6 16-40, E6 31-55, and E6 106-130 regions. Positive responses were seen in the E6 1-25, E6 106-130, E6 136-158, and E7 1-25 regions in the post-2 vaccination sample, and in the E6 31-55, E6 91-115, and E7 46-70 regions in the post-4 vaccination sample. The increase in the response to the HPV16 E6 91-115 regions was statistically significant (paired t-test, p=0.023) after 4 vaccinations. Phytohemagglutinin was used as a positive control (not shown). The y-axis represents mean spot forming units of triplicates per 1 x 106 CD3+ T cells, and error bars represent standard error of means. (C) The fluorescent cell sorter analysis of PBMCs revealed that the Th1 (CD4+Tbet+) level expressed as the percentage of CD4+ T cells increased after 4 vaccinations, but Treg (CD4+CD25+FoxP3+) and Th2 (CD4+GATA3) levels were minimally changed.