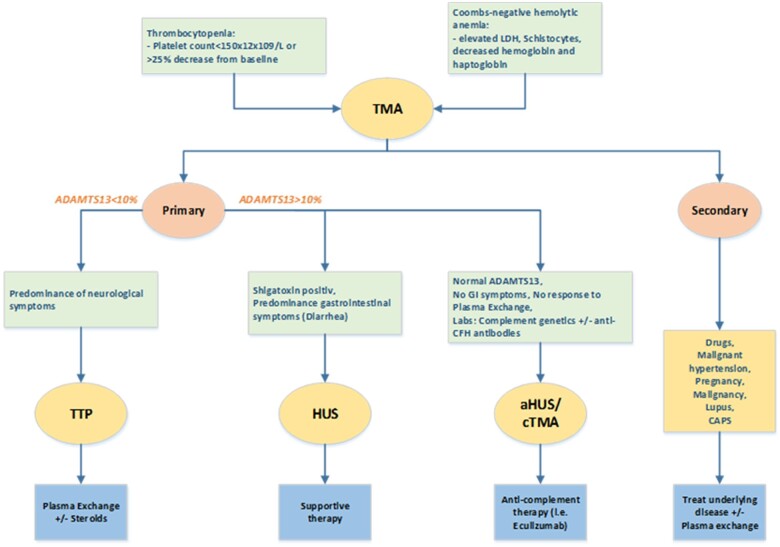
Figure 2.
Diagnostic algorithm for thrombotic microangiopathies. Thrombocytopenia and Coombs-negative anaemia suggest for a diagnosis of thrombotic microangiopathy. Thrombotic microangiopathy is divided in primary and secondary. The first includes a spectrum of entities like thrombocytopenic purpura, haemolytic uraemic syndrome, and complement-mediated thrombotic microangiopathy. The main characteristics of thrombocytopenic purpura is ADAMTS13 reduction or deficiency and neurological syndrome. In haemolytic uraemic syndrome, the prominent organ injury is renal and ADAMTS13 activity is normal. Typical haemolytic uraemic syndrome is caused from enteric bacterial infection (Escherichia coli) that produces Shigatoxin that damages endothelial cells. More rarely, there are thrombotic microangiopathies cases with normal ADAMTS13 activity and in the absence of Shiga toxin-producing organisms or enteric symptoms. This group is referred to complement-mediated thrombotic microangiopathy (or atypical haemolytic uraemic syndrome) due to the dysregulation of the alternate complement pathway leading to complement overactivation and consequent endothelial damage. Secondary thrombotic microangiopathies are caused by other underlying conditions, such as infections, autoimmune disease, or medical treatment. ADAMTS13, a disintegrin and metalloprotease with thrombospondin type 1 motifs, member 13; CFH, complement factor H; cTMA, complement-mediated thrombotic microangiopathy; GI, gastrointestinal; HUS, haemolytic uraemic syndrome; TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura.