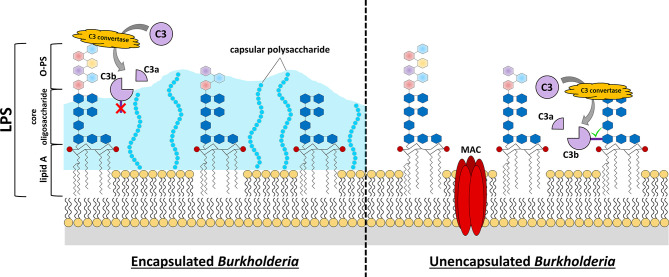
Figure 1.
Burkholderia cell surface structures involved in evading complement-mediated killing. C3 convertase-mediated cleavage of C3 into C3a and C3b reveals an unstable thioester bond on C3b (dark purple rectangle). If this protein is not generated close to the cell surface or other receptive structures, the thioester bond is quickly hydrolyzed, and the protein loses its enzymatic activity or its ability to attach as an opsonin to the surface (red “X”, C3b in left panel). Many pathogenic Burkholderia express capsular polysaccharide that extends quite distant to the cell membrane. This can provide protection from complement activation and opsonization because its structure is not conducive to C3b-binding and, if binding does occur, the bound C3b is relatively distant from the bacterial cell membrane to allow MAC formation. While the lipid A and core oligosaccharide structures of Burkholderia LPS are fairly conserved, there is considerable variation in the O-PS of these bacteria (multi-colored hexagons). Analysis of LPS structures of representative Burkholderia spp. have demonstrated that these organisms harbor 4-amino-4-deoxy-L-arabinose (Ara4N) modifications on the phosphate groups (red circles) of the lipid A disaccharide backbone. The expression of elongated O-PS moieties stretching away from the cell surface can protect against complement-mediated killing by preventing C3 convertase formation close to the cell surface, and thus prevent insertion of a MAC complex in the cell membrane. If C3 convertase forms close to the cell membrane (e.g. LPS core moieties, etc.), the exposed thioester of C3b quickly binds nearby hydroxyl or amino groups (green check mark, C3b in right panel). Complement opsonins that bind distant from the cell surface can promote killing by opsonophagocytosis if they can be recognized by phagocytic cells, however activation/binding near the surface promotes direct killing via formation of MAC and bacterial lysis.