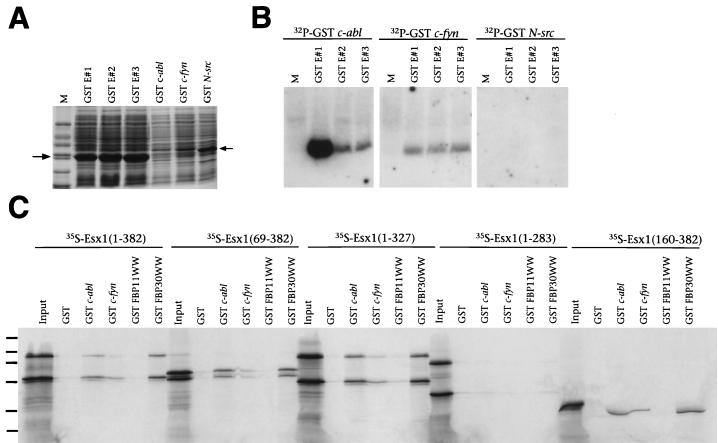
FIG. 7.
The proline-rich region of ESX1 confers binding to SH3 and WW domains. (A) SDS-PAGE analysis of total-cell lysates containing GST ESX1 fusion peptides (GST E#1, GST E#2, or GST E#3) and GST SH3 domain fusion proteins (GST c-abl, GST c-fyn, or GST N-src), visualized by staining with Coomassie blue. Markers (M) are 66, 45, 36, 24, 20, and 14 kDa. (B) Far-Western analysis of the interaction of GST ESX1 fusion peptides with radiolabeled SH3 domains. Each lane contains equal amounts (15 μg) of a total-cell lysate containing GST ESX1 fusion peptides (GST E#1, GST E#2, or GST E#3) probed with the indicated radiolabeled GST SH3 domain fusion proteins. M denotes the lane containing molecular mass markers. (C) GST interaction assays with radiolabeled in vitro-translated ESX1 proteins (input), followed by incubation with GST alone, with GST fused to SH3 domains (c-abl or c-fyn), or with GST fused to WW domains (FBP11WW or FBP30WW). Proteins were visualized by SDS-PAGE followed by autoradiography. Molecular mass standards (130, 90, 66, 45, 36, and 24 kDa) are indicated by dashes.