Fig 1. Overview of LLPS, phase diagram, and modulation.
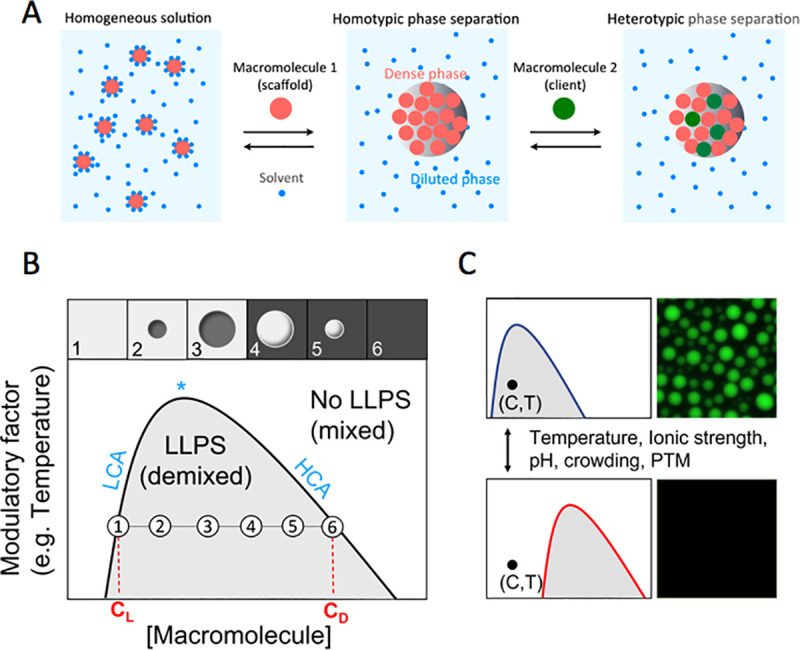
(A) Under certain conditions, a solvated macromolecule (scaffold) undergo homotypic LLPS and concentrate in a distinct liquid compartment (dense phase). One or more additional macromolecules (clients) can partition into the new phase through heterotypic LLPS. (B) A phase diagram describes the phase behavior of a binary (macromolecule and solvent) or multicomponent system (at least 2 macromolecules) as a function of macromolecular concentration or any other physicochemical factor that may modulate its condensation tendency. Here, we present a concentration vs temperature phase diagram for a binary system. A phase boundary (black curve, known as binodal) defines whether the system is in a 1-phase regime (mixed solution) or in a 2-phase regime (demixed solution). All coordinate pairs of concentration and temperature that lie beneath the phase boundary (gray) give rise to LLPS. The phase boundary maximum is the critical point (star), above which a homogeneous solution is seen at any macromolecular concentration. The critical point divides the phase boundary in 2 segments known as low concentration arm (LCA, left) and high concentration arm (HCA, right). The LCA defines the concentration of the diluted or light phase (CL), whereas the HCA defines the concentration of the dense phase (CD). Increasing total concentration in the light phase above the concentration threshold only changes the relative volumes between phases (i.e., droplets become larger at the expense of the diluted phase; see Fig 3). The top panel of (B) illustrates this phenomenon: (1) macromolecule concentration at threshold, no LLPS. At higher concentration, small droplets form (2) growing in size (3) as concentration increases. Eventually, the volume of the dense phase is higher than the diluted phase, so surface tension dictates the formation of diluted droplets surrounded by dense phase (4). After this inversion boundary, increasing concentration decreases the diluted droplets size (5) until a 1-phase regime of dense solution only is achieved (6). (C) Modulatory effectors such as PTMs or pH operate by altering the forces that drive droplet formation, thus changing the phase boundary. All of these modulatory effects may act in favor or against LLPS, depending on the nature of the interactions involved. For instance, addition of a negative charge by phosphorylation has the potential to engage components in electrostatic attractive or repulsive forces. C,T, concentration, temperature; HCA, high concentration arm; LCA, low concentration arm; LLPS, liquid–liquid phase separation; PTM, posttranslational modification.
