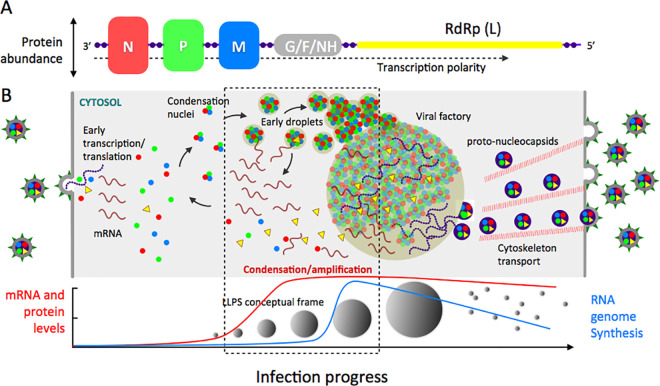
Fig 2. A general integrative model for condensation of VFs based on NSVs.
(A) Simplified depiction of nsNSV genome structure. The viral transcription mechanism ensures a gradient of protein expression, with higher abundance of N, encoded at the 3′ region, and lower abundance of the large polymerase, invariably encoded at the 5′ end of nsNSV genomes. (B) Model depicting the formation of cytosolic VFs along viral infection. The schema stresses the amplification effect on RNA and protein synthesis caused by the self-primed formation of condensates (see main text). Toward the end of the infectious cycle, the immature nucleocapsids (proto-nucleocapsids) protrude from the VF and are transported along the cytoskeleton network to the assembly site [73]. The principle behind LLPS indicate that as the concentration of components increase, a partition into a newer denser phase takes place, where the concentration within the droplets remain constant, and further increase in the concentration of the components in the surrounding milieu cause an increment of the size of the condensates. The condensation event causes an abrupt increase of the effective local concentration of RNA and proteins, the drivers of the LLPS, which results in a substantial amplification of transcription and genome replication in the VF. LLPS, liquid–liquid phase separation; nsNSV, nonsegmented NSV; NSV, negative-stranded virus; RdRp, RNA-dependent RNA polymerase; VF, viral factory.