FIGURE 5.
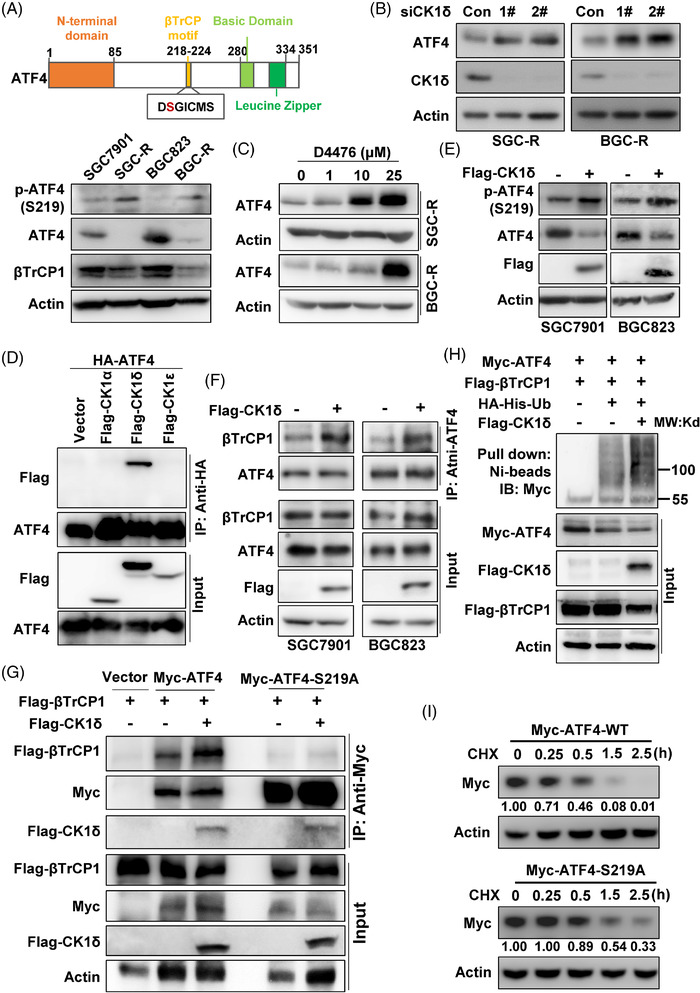
CK1δ phosphorylates ATF4 to stimulate its ubiquitination‐dependent proteasomal degradation. (A) The scheme of ATF4 protein was shown, and expression of phosphorylated ATF4‐S219 [p‐ATF4 (S219)], total ATF4 and βTrCP1 in sensitive and resistant cells were determined with western blotting. Expression of ATF4 in SGC‐R (left) or BGC‐R (right) with CK1δ knockdown (B) or inhibition (C) by D4476 was detected by western blotting. (D) Co‐IP was performed with anti‐HA antibody in HEK293T cells with HA‐ATF4 and Flag‐CK1α/δ/ε co‐transfection, and the interaction was detected by western blotting with anti‐Flag and anti‐ATF4 antibodies. (E) p‐ATF4(S219) in SGC7901 (left) or BGC823 (right) with Flag‐CK1δ over‐expression was analysed by western blotting. (F) After Flag‐CK1δ transfection, cells were pre‐treated with MG132, and interaction of ATF4 with βTrCP1 in SGC7901 (left) or BGC823 (right) cells was analysed by anti‐ATF4 co‐IP, followed by anti‐βTrCP1 and anti‐ATF4 immunoblot. (G) Interaction of wild‐type Myc‐ATF4 (Myc‐ATF4‐WT) or Myc‐ATF4‐S219A mutant with Flag‐βTrCP1 with/without Flag‐CK1δ co‐transfection in HEK293T cells was determined with anti‐Myc co‐IP, followed by anti‐Flag and anti‐Myc immunoblot. (H) βTrCP1‐mediated ATF4 ubiquitination in HEK293T cells with/without Flag‐CK1δ over‐expression was measured by in vitro ubiquitination assay. (I) Protein turnover of exogenous Myc‐ATF4‐WT or Myc‐ATF4‐S219A in SGC‐R cells under CHX (50 μg/ml) treatment with indicated times was detected by western blotting using anti‐Myc antibody. The relative grey value of ATF4 compared to Actin was analysed, and the normalised expression ratio was shown
