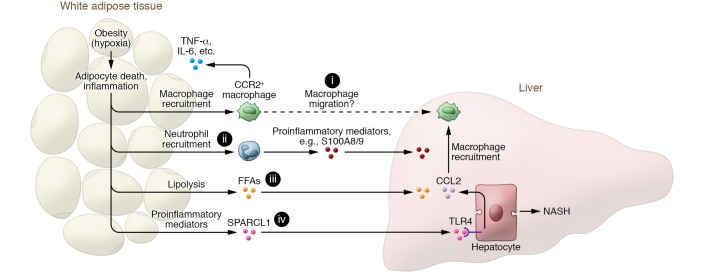
Figure 1. Adipose tissue inflammation promotes NASH progression via proinflammatory mediators, including SPARCL1.
Adipocyte hypertrophy during obesity can cause adipose tissue hypoxia and subsequent adipocyte death and adipose tissue inflammation, which further promote the recruitment of macrophages and neutrophils in adipose tissue. The infiltrated CCR2+ macrophages produce cytokines (such as TNF-α, IL-6, etc.) that perpetuate adipose tissue inflammation and promote hepatic macrophage infiltration (i) and liver inflammation. Recruitment of neutrophils in adipose tissue (ii), which is dependent on E-selectin, accelerates NASH progression by producing many inflammatory mediators (such as S100A8 and S100A9). In addition, adipose tissue inflammation activates adipocyte lipolysis to release free fatty acids (FFAs) (iii), leading to liver injury and inflammation. Finally, dysregulated adipose tissue can produce many proinflammatory mediators, including SPARCL1 (iv) whose function in NASH progression is reported by Liu, Xiang, et al. (22). Notably, SPARCL1 stimulates hepatocytes to produce CCL2 that induces hepatic macrophage recruitment and liver inflammation, thereby promoting NASH progression. Inhibition of SPARCL1 has therapeutic potential for the treatment of NASH.