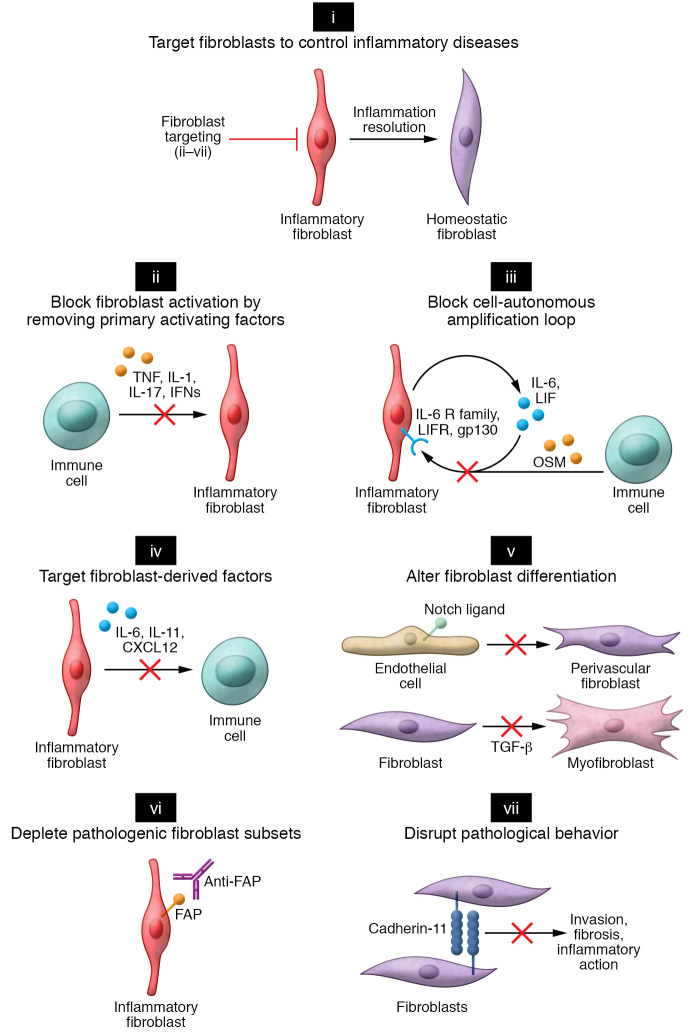
Figure 3. Approaches to targeting fibroblasts in inflammatory diseases.
(i) Strategies to target fibroblasts in inflammatory disease can be categorized into several broad categories. (ii) Preventing fibroblast activation by neutralizing key cytokines such as TNF, IL-1, IL-17, and IFNs that lead to fibroblast inflammatory activation. (iii) Primary activating factors. The full extent of fibroblast inflammatory activation requires secreted autocrine factors (LIF) and paracrine factors (OSM). Strategies to block fibroblast amplification factors could result in attenuating the fibroblast inflammatory response. (iv) Targeting key fibroblast-derived factors crucial for immune cell activation or recruitment. (v) Preventing pathogenic fibroblast differentiation by blocking morphogen signaling pathways. In RA, Notch-mediated vascular fibroblasts could be targeted by blockade of Notch3 receptor signaling. In fibrotic disease, myofibroblast differentiation driven by TGF-β could be targeted by blockade of TGF-β signaling. (vi) Depleting pathogenic fibroblast subsets. Conceptually, pathogenic fibroblasts in inflammatory disease expressing distinct surface markers, such as fibroblast activation protein (FAP), could be targeted therapeutically through an antibody-mediated depletion strategy. Markers specifically expressed in disease-associated fibroblasts would enable depletion of pathogenic subsets without disruption of normal fibroblast functions in noninvolved organ tissues. (vii) Disrupting pathological processes mediated by fibroblasts. In this example, an inhibitory antibody against cadherin-11 could potentially prevent fibroblast-mediated invasion and blunt cytokine-induced inflammation.