Abstract
Background
There is ongoing debate about the possible protective effect of the bivalent human papillomavirus (2vHPV) vaccine, targeting oncogenic types HPV-16/18, against anogenital warts (AGWs), commonly attributed to HPV-6/11. We performed a retrospective registry-based open cohort study to assess the effect of 2vHPV vaccination on AGWs.
Methods
We linked general practice (ie, primary care) data from women born between 1993 and 2002, who had been eligible for HPV vaccination in the Netherlands, to the Dutch national immunization registry on an individual level. Women were followed until their first AGW diagnosis or end of follow-up. Adjusted incidence rate ratios (aIRRs) were estimated using Poisson regression with vaccination status as a time-dependent exposure.
Results
We linked data of 96 468 women with a total of 328 019 years observation time and 613 AGW diagnoses (incidence: 1.87/1000 person-years). At the end of follow-up, 61% were 2vHPV vaccinated (≥ 1 dose) of whom 91% were fully vaccinated. The AGW incidence was lower among those with ≥ 1 dose vs 0 doses (aIRR, 0.75 [95% confidence interval {CI}, .64–.88]). The effect of vaccination was stronger after full vaccination (aIRR, 0.72 [95% CI, .61–.86]) and for women who were offered vaccination at 12–13 years of age (aIRR, 0.69 [95% CI, .51–.93]) vs those at 13–16 years of age (aIRR, 0.77 [95% CI, .64–.93]).
Conclusions
This is the largest population-based study so far to examine the effect of 2vHPV vaccination on AGWs, with reliable individual information on AGW diagnoses and vaccination status. The results indicate that 2vHPV vaccination partially protects against AGWs, especially when administered in early adolescence.
Keywords: human papillomavirus, bivalent HPV vaccine, anogenital warts, vaccine effectiveness, cross-protection
In this large population-based cohort study linking primary care and immunization registry data, anogenital wart incidence was significantly reduced among human papillomavirus (HPV) type 16/18–vaccinated vs unvaccinated women, suggesting a partial protective effect of bivalent HPV vaccination against anogenital warts.
Human papillomavirus (HPV) is a highly contagious sexually transmitted virus and associated with the development of various cancers, recurrent respiratory papillomatosis, and anogenital warts (AGWs) [1–3]. Although AGWs are not life-threatening, the burden and associated treatment costs are substantial [4]. In industrialized countries, the annual AGW incidence is 0.1%–0.2%, with a peak occurring in women younger than 24 years of age [5]. Women with AGW can experience mild symptoms such as pain, itch, and discharge from the urethra or vagina. Moreover, AGWs can have a negative impact on psychosocial well-being [6, 7]. Treatment of AGWs is focused on the removal of visible warts and not on elimination of the underlying HPV infection, leading to high recurrence rates (up to 80%) [8].
HPV types 6 and 11 are believed to cause the vast majority of AGWs [2]. Currently, 2 prophylactic vaccines are available that target HPV-6/11 to prevent AGWs: the quadrivalent (4vHPV) vaccine and, since 2015, the nonavalent (9vHPV) vaccine. Countries that have implemented these vaccines in their national immunization program (NIP) with high vaccination coverage have observed drastic declines in AGWs [9].
In the NIP of the Netherlands, the bivalent HPV (2vHPV) vaccine is used, which targets HPV-16/18. The program started in 2009 with a one-off catch-up campaign for girls born in 1993–1996. Routine HPV vaccination started in 2010, offering vaccination to girls in the year they turn 13 years old, beginning with birth cohort 1997. Initially, vaccination was offered as a 3-dose schedule (0, 1, and 6 months), but in 2014 the program changed to a 2-dose schedule (0 and 6 months) [10].
Even though the 2vHPV vaccine does not target HPV-6/11, there is ongoing debate about the possible protective effect of 2vHPV vaccination against AGWs. After introduction of 2vHPV vaccination in the United Kingdom, a small but prominent decrease in AGW diagnoses was observed among young women at genitourinary medicine clinics, while such a decline was not observed for other sexually transmitted infections (STIs). The authors concluded that at least part of this decline was likely due to 2vHPV vaccination, and they calculated a vaccine effectiveness of 34% [11]. Other studies from the United Kingdom, the Czech Republic, and Spain did not find evidence for an effect of 2vHPV vaccination on AGWs [12–14]. In a previous study among visitors of sexual health centers in the Netherlands, we found a lower AGW prevalence among self-reported vaccinated compared with self-reported unvaccinated women, albeit statistically nonsignificant [15]. This study had low numbers of AGWs, partly because 95% of all AGW diagnoses in the Netherlands are made by general practitioners (GPs) [16].
Knowledge about the protective effect of 2vHPV vaccination against AGWs is important for comprehensive cost-effectiveness analyses and evidence-based communication strategies. Because the Netherlands is one of the few industrialized countries that have consistently used the 2vHPV vaccine in the NIP, we had the unique opportunity to study this effect in the general population. By linking patient data from GPs with data from the national immunization registry, we performed a retrospective registry-based open cohort study to assess the effect of 2vHPV vaccination on the incidence of AGWs diagnosed by GPs in the Netherlands.
METHODS
Study Design and Population
The study population was constructed retrospectively by linking individual data from 3 data sources: (1) the Nivel Primary Care Database (Nivel-PCD) of GPs; (2) the national immunization registry (Præventis); and (3) the Dutch population registry (Supplementary File 1). The study population included women born between 1993 and 2002 who were registered in a general practice (ie, primary care) participating in the Nivel-PCD at some point during 2007–2015 and who were invited for HPV vaccination through the NIP. This was an open cohort, in which women could leave a general practice and re-enter later.
Data Sources
Nivel-PCD
The Nivel-PCD provides a representative sample of general practices with respect to geographical distribution and urbanization degree and a representative sample of about 10% of the Dutch population with respect to age and sex [17]. In the Netherlands, in principle, all inhabitants register in a general practice to have access to the healthcare system, irrespective of having healthcare consultations. GPs participating in the Nivel-PCD provide routinely recorded data from electronic medical files, including registration of patients and data on diagnoses (using International Classification of Primary Care, first edition [ICPC-1] codes) and prescriptions (using Anatomical Therapeutic Chemical Classification System [ATC] codes). We restricted our analysis to data from general practices with complete (≥ 46 weeks data per year) and good-quality data (≥ 70% of records with valid codes).
The period a woman was registered in a general practice, based on quarterly claim data, was used as the time-at-risk for AGW diagnoses. If a gap in registration was half a year or longer, we assumed that the patient left the general practice temporarily and this unregistered period was not included in the analyses. Gaps in the registration period of 1 quarter were ignored (Supplementary File 2).
We obtained data of consultations with the diagnosis AGW (ICPC-1 code X91). As a proxy for sexual risk behavior, we used a consultation coded as fear of STIs (ICPC-1 code X23) or as fear of human immunodeficiency virus (HIV)/AIDS (ICPC-1 code B25). The mean number of GP consultations per year during follow-up was used as a proxy for health-seeking behavior. Finally, we collected data on consultations with the prescription 4vHPV vaccine (ATC code J07BM01) or 2vHPV vaccine (ATC code J07BM02).
Præventis
Præventis is the national electronic immunization registry including all vaccinations administered as part of the NIP [18]. We obtained individual data on whether someone was invited for HPV vaccination, the number and date of received HPV vaccine doses, and the type of vaccine given (2vHPV, 4vHPV, or unknown).
Dutch Population Registry
From the national population registry, we obtained data on the month and year of birth, migration background, and educational level. The day of birth was set to 15 for all women. Migration background was based on (parental) country of birth [19]. Educational level was based on the highest registered education a woman completed or was following as of December 2015. We categorized the educational level as high (school of higher general secondary education, pre–university education, university of applied sciences, and university), or middle/low (all other forms of education).
The 3 data sources were linked using a unique identification number allocated by a trusted third party (Statistics Netherlands), based on either the encrypted citizen service number or a combination of sex, date of birth, and 4 digits of the postal code. Data extraction from Nivel-PCD has been approved according to the Nivel-PCD governance code under number NZR-00316.016. The study was exempt from formal medical-ethical approval under prevailing laws in the Netherlands as it concerned a retrospective observational study using de-identified data only. All parties granted approval for the usage and linkage of the data.
Statistical Analyses
Women entered the cohort on 1 January 2007, on the date they were first registered in the GP’s database, or on their 12th birthday, whichever came last. We excluded women who were HPV vaccinated in a general practice (outside the NIP), could not be matched to the other databases, or received 4vHPV or an unknown HPV vaccine as registered in Præventis. Women were followed until the first of the following: end of study period (31 December 2015), first AGW diagnosis registered during the follow-up period, or date of leaving the GP’s database (end of follow-up). Only the first AGW diagnosis was included, because of the inability in this registry-based study to differentiate additional consultations as recurrent AGW diagnoses or as repeated consultations for the same wart. Observation time and events following the first AGW diagnoses were censored.
We calculated the adjusted incidence rate ratio (aIRR) of AGWs after being HPV vaccinated (≥ 1 dose) relative to being unvaccinated, using Poisson regression with a log link function, log observation time as offset, and robust standard errors. Moreover, we made a distinction between being fully vaccinated (3 doses or 2 doses ≥ 5 months apart, regardless of year of vaccination) or partially vaccinated (1 dose or 2 doses < 5 months apart). Vaccination status was a time-dependent exposure, meaning that a woman could contribute observation time to the unvaccinated and the vaccinated category if she received vaccination during follow-up (Supplementary File 3). All analyses were adjusted for age as a time-dependent variable. In addition, we adjusted for migration background, educational level, having had a fear of STI/HIV consultation, and mean number of GP consultations per year. We also performed the analyses separately for birth cohorts 1996–2002 (cohorts who were offered vaccination according to the routine program at 12–13 years of age) and birth cohorts 1993–1995 (cohorts who were 13–16 years old when vaccination was offered).
The incubation period for AGWs is estimated to be between 3 and 18 months with a median of 6–10 months [4]. Because HPV vaccination has no effect on HPV types present at the time of vaccination [20], in sensitivity analyses we added buffer periods of 3, 6, and 10 months to the vaccination date to account for prevalent infections at the time of vaccination (Supplementary File 4). We also repeated the analyses excluding women with gaps in observation time.
The statistical analyses were performed using RStudio version 1.1.463 (RStudio, Boston, Massachusetts) with a statistical significance level of P < .05. Records with missing data were excluded from the analyses, as they represented < 5% of the study population.
RESULTS
Study Population
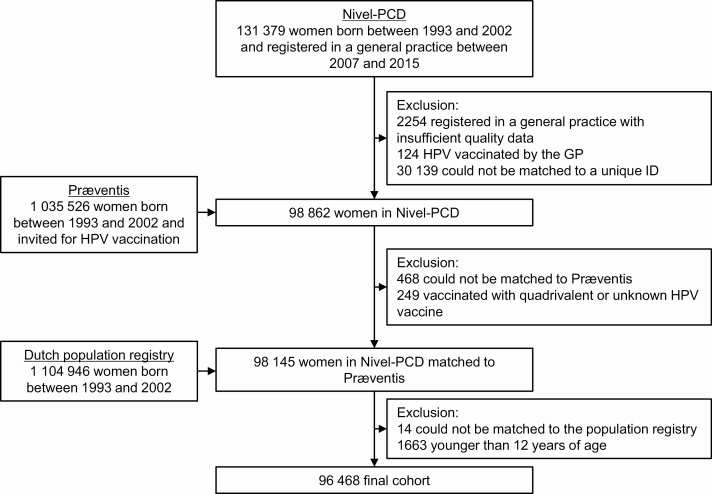
In total, 131 379 women born between 1993 and 2002 were registered in a general practice participating in the Nivel-PCD between 2007 and 2015, of whom 129 125 (98%) were registered in a practice with complete and good-quality data. After applying all exclusion criteria, 96 468 women (73%) were included in the final cohort. The majority of the women who were excluded could not be matched to the other databases; a limited number of women were excluded because they were vaccinated by the GP (n = 124) or with the 4vHPV or unknown vaccine (n = 249) (Figure 1). Together, the women contributed 328 019 years of observation time (median, 3 years per woman). Table 1 presents the characteristics of the study population.
Figure 1.
Study flowchart. Abbreviations: GP, general practitioner; HPV, human papillomavirus; ID, identifier; Nivel-PCD, Nivel Primary Care Database (network of representative general practices across the Netherlands); Præventis, national electronic immunization registry.
Table 1.
Characteristics of the Study Population That Was Included in the Analyses
| General Practitioner | Total Study Population (N = 96 468) | |
|---|---|---|
| No. | (%) | |
| Observation time in years per womana | ||
| Median (25th–75th percentile) | 3.0 (2.0–5.0) | … |
| Vaccination status at the start of follow-up | ||
| Unvaccinated | 67 603 | (70.1) |
| Partially vaccinatedb | 2783 | (2.9) |
| Fully vaccinatedb | 26 082 | (27.0) |
| Vaccination status at the end of follow-up | ||
| Unvaccinated | 37 675 | (39.1) |
| Partially vaccinatedb | 5049 | (5.2) |
| Fully vaccinatedb | 53 744 | (55.7) |
| Age, y | ||
| Range | 12.00–22.96 | … |
| Median (25th–75th percentile) at the start of follow-up | 13.4 (12.0–16.1) | … |
| Median (25th–75th percentile) at the end of follow-up | 17.7 (15.3–20.1) | … |
| Migration backgroundc | ||
| Native Dutch | 75 595 | (78.4) |
| Moroccan | 2994 | (3.1) |
| Turkish | 2981 | (3.1) |
| Surinamese | 2249 | (2.3) |
| Antillean/Aruban | 1139 | (1.2) |
| Western (non-Dutch) | 6158 | (6.4) |
| Non-Western (other than above) | 5352 | (5.5) |
| Educational leveld | ||
| Low/middle | 50 537 | (52.4) |
| High | 44 563 | (46.2) |
| Missing | 1368 | (1.4) |
| Fear of STI or fear of HIV/ AIDS consultatione | ||
| No | 92 753 | (96.1) |
| Yes | 3715 | (3.9) |
| Mean No. of GP consultations per year | ||
| Median (25th–75th percentile) | 2.2 (0–4.0) | … |
Abbreviations: GP, general practitioner; HIV, human immunodeficiency virus; STI, sexually transmitted infection.
aObservation time was defined as the period a woman was registered in a general practice. Follow-up started the first time a woman was registered in a general practice from January 2007 onward or on the 12th birthday. Follow-up ended when a woman was diagnosed with anogenital warts, left the general practice, or end of the study period (31 December 2015).
bPartially vaccinated: 1 dose or 2 doses < 5 months apart. Fully vaccinated: 3 doses or 2 doses ≥ 5 months apart.
cMigration background was based on parental country of birth.
dHigh educational level included school of higher general secondary education, pre–university education, university of applied sciences, and university. Low/middle educational level included all other levels of education.
e International Classification of Primary Care, first edition, code X23 or B25 (fear of STI or fear of HIV/AIDS, respectively) prior to an anogenital wart diagnosis.
In total, 28 865 women (29.9%) were already vaccinated at least once when entering the cohort and 30 609 women (31.7%) were vaccinated with at least 1 dose during follow-up. At the end of follow-up, 58 793 women (60.9%) were vaccinated with the 2vHPV vaccine (≥ 1 dose), 53 744 (55.7%) were fully vaccinated, and 5049 (5.2%) were partially vaccinated.
Anogenital Warts
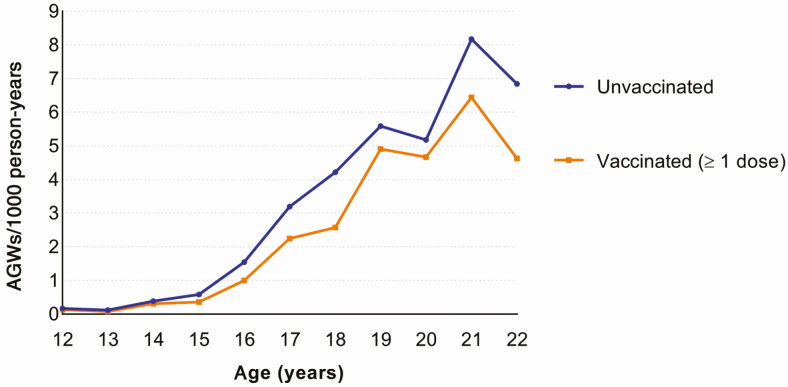
In total, 613 women had an AGW diagnosis registered by a GP during the follow-up period (overall incidence, 1.87/1000 person-years). The AGW incidence increased steadily with age, with a peak at 21 years of age (7.21/1000 person-years). The incidence of AGW was lower for vaccinated compared to unvaccinated women across all ages (Figure 2).
Figure 2.
Incidence of anogenital warts (AGWs) by age and vaccination status.
The AGW incidence was lower after ≥ 1 dose of 2vHPV vaccine relative to 0 doses (aIRR, 0.75 [95% confidence interval {CI}, .64–.88]) (Table 2). The effect was stronger after being fully vaccinated (aIRR, 0.72 [95% CI, .61–.86]) and not observed after being partially vaccinated (aIRR, 0.96 [95% CI, .68–1.32]), although the difference between fully and partially vaccinated itself was not statistically significant (P = .1).
Table 2.
Association Between Bivalent Human Papillomavirus Vaccination and Anogenital Warts Diagnosed by General Practitioners
| No.a | Observation Time, y | AGW Diagnoses | aIRRb (95% CI) | aIRRc (95% CI) | |
|---|---|---|---|---|---|
| Unvaccinated | 66 487 | 144 129 | 296 | Reference | Reference |
| Vaccinated (≥1 dose) | 58 299 | 180 497 | 310 | 0.76 (.65–.89) | 0.75 (.64–.88) |
| Unvaccinated | 66 487 | 144 129 | 296 | Reference | Reference |
| Partially vaccinatedd | 31 790 | 26 409 | 42 | 1.15 (.82–1.57) | 0.96 (.68–1.32) |
| Fully vaccinatedd | 53 389 | 154 088 | 268 | 0.72 (.61–.85) | 0.72 (.61–.86) |
Abbreviations: AGW, anogenital warts; aIRR, adjusted incidence rate ratio; CI, confidence interval; GP, general practitioner.
aNumber of women that contributed observation time per vaccination status. One woman could contribute observation time to > 1 vaccination status. Women with a missing educational level were excluded.
bAdjusted for age as time-varying.
cAdjusted for age as time-varying, migration background, educational level, fear of sexually transmitted infection/human immunodeficiency virus consultations, and mean number of GP consultations per year.
dPartially vaccinated: 1 dose or 2 doses < 5 months apart. Fully vaccinated: 3 doses or 2 doses ≥ 5 months apart.
Overall, 67 179 women (70%) were born between 1996 and 2002 (12–13 years of age when vaccination was offered) and 29 289 (30%) were born between 1993 and 1995 (13–16 years of age when vaccination was offered). The aIRR of ≥ 1 dose relative to 0 doses was 0.69 (95% CI, .51–.93) for the younger birth cohorts and 0.77 (95% CI, .64–.93) for the older birth cohorts. After a full vaccination course, these figures were 0.60 (95% CI, .44–.83) and 0.77 (95% CI, .63–.93), respectively (Table 3).
Table 3.
Association Between Bivalent Human Papillomavirus Vaccination and Anogenital Warts Diagnosed by General Practitioners, Stratified by Birth Cohort
| Vaccination Status | No.a | Observation Time, y | AGW Diagnoses | aIRRb (95% CI) | aIRRc (95% CI) |
|---|---|---|---|---|---|
| Cohorts 1996–2002d | |||||
| Unvaccinated | 51 454 | 91 815 | 85 | Reference | Reference |
| Vaccinated (≥ 1 dose) | 41 921 | 116 874 | 93 | 0.68 (.51–.91) | 0.69 (.51–.93) |
| Unvaccinated | 51 454 | 91 815 | 85 | Reference | Reference |
| Partially vaccinatede | 28 022 | 20 219 | 20 | 1.99 (1.18–3.19) | 1.47 (.86–2.40) |
| Fully vaccinatede | 38 381 | 96 655 | 73 | 0.58 (.42–.79) | 0.60 (.44–.83) |
| Cohorts 1993–1995d | |||||
| Unvaccinated | 15 033 | 52 314 | 211 | Reference | Reference |
| Vaccinated (≥ 1 dose) | 16 378 | 63 623 | 217 | 0.79 (.65–.95) | 0.77 (.64–.93) |
| Unvaccinated | 15 033 | 52 314 | 211 | Reference | Reference |
| Partially vaccinatede | 3768 | 6190 | 22 | 0.92 (.57–1.39) | 0.80 (.50–1.22) |
| Fully vaccinatede | 15 008 | 57 433 | 195 | 0.78 (.64–.94) | 0.77 (.63–.93) |
Abbreviations: AGW, anogenital warts; aIRR, adjusted incidence rate ratio; CI, confidence interval.
aNumber of women who contributed observation time per vaccination status. One woman could contribute observation time to > 1 vaccination status. Women with a missing educational level were excluded.
bAdjusted for age as time-varying.
cAdjusted for age as time-varying, migration background, educational level, fear of sexually transmitted infection/human immunodeficiency virus consultations, and mean number of general practitioner consultations per year.
dBirth cohorts 1996–2002 were 12–13 years old when vaccination was offered. Birth cohorts 1993–1995 were 13–16 years old when vaccination was offered.
ePartially vaccinated: 1 dose or 2 doses < 5 months apart. Fully vaccinated: 3 doses or 2 doses ≥ 5 months apart.
Sensitivity Analyses
In both sensitivity analyses, results were not meaningfully different from the main analyses (Supplementary File 5). With a buffer period of 10 months, the effect of vaccination was slightly smaller (aIRR for ≥ 1 dose, 0.77 compared to 0.75 in the main analyses), whereas the effect was slightly larger (aIRR for ≥ 1 dose, 0.70) when only women without gaps in observation time (n = 87 612 [91%]) were included.
DISCUSSION
We studied the effect of 2vHPV vaccination on AGWs diagnosed by GPs in the Netherlands by linking GP data to the vaccination registry. We showed a significantly reduced incidence of AGWs among 2vHPV vaccinated women compared to unvaccinated women. The effect of vaccination was stronger after completion of the vaccination course and for women offered vaccination at 12–13 years of age.
To our knowledge, this is the largest study so far to assess the direct effect of 2vHPV vaccination on clinically relevant AGWs. To minimize bias, we used population-based data with reliable vaccination status and AGWs diagnosed at the general practice, where about 95% of all AGW diagnoses in the Netherlands are made [16]. We do acknowledge some limitations. First, not all women in the GP database could be linked to the vaccination registry. Comparing our study population to all women born between 1993 and 2002 living in the Netherlands, our study population was slightly more often native Dutch (78% vs 71%), highly educated (46% vs 43%), and HPV vaccinated (62% vs 58% as of December 2015). This suggests that our study population might not have been fully representative for the total Dutch population. Because a native Dutch background and high educational level were associated with a lower risk for AGWs, we might have underestimated the overall AGW incidence, but this effect is likely to be small. Second, women were defined to enter or leave the cohort depending on registration in a general practice. Therefore, women might have already been diagnosed with AGWs before entering the cohort. Assuming nondifferential misclassification between vaccinated and unvaccinated women, this would lead to an underestimation of the protective effect of vaccination. Misclassification of vaccination status is unlikely, because virtually all vaccinations are registered in Præventis (as demonstrated in our study: only 0.1% were vaccinated by the GP).
The preferred design to measure a causal direct effect of vaccination would be a randomized placebo-controlled trial (RCT), but RCTs of the 2vHPV vaccine did not include AGWs among reported outcomes [21]. Performing an RCT now would be unethical, because of the already demonstrated very high efficacy against oncogenic HPV types and associated precancer lesions [22]. Investigation into the effect of 2vHPV vaccination on AGWs therefore relies on large observational studies with minimal risk of bias. However, we cannot rule out residual confounding, implying that the lower observed incidence of AGWs among 2vHPV-vaccinated women could be related to an overall lower risk of AGWs among vaccinated compared to unvaccinated women, unrelated to vaccination itself. We had limited information on possible confounders to fully adjust for this. Previous studies did observe small differences in risk behavior between HPV-vaccinated and unvaccinated women in the Netherlands, but these variables hardly affected the association between vaccination and HPV outcome measures, suggesting limited confounding [23].
An explanation for the observed lower incidence of AGWs among 2vHPV vaccinated women is that 2vHPV vaccination also effectively protects against AGWs. This supposition is supported by a number of observations. First, we observed a dose-response relation, with stronger effects after a complete vaccination course compared to an incomplete vaccination course. Similar results were observed elsewhere for the 4vHPV vaccine against AGWs [24]. Second, the effect of vaccination was lower for the older birth cohorts in our analysis. This is in line with the observed lower vaccine effectiveness among women with HPV exposure prior to vaccination, which is more likely if vaccination is offered at an older age [22]. Last, our results were very robust, with an effect of 2vHPV vaccination that was consistent across all ages and not affected by considerations of a buffer period or complete observation time.
The biological mechanism behind the apparent effectiveness of 2vHPV vaccination against AGWs is not clear. One possible explanation is that high-risk HPV types play a larger role in the development of AGWs than generally assumed [25]. High-risk HPV types, including the 2vHPV vaccine types HPV-16/18, are frequently genotyped in AGWs (generally ranging between 30% and 40%; in some AGWs, only high-risk HPV types are genotyped) [2, 26–29]. Because the 2vHPV vaccine induces excellent effectiveness against HPV-16/18 and considerable cross-protection against several other high-risk types [30–32], a putative causative contribution of high-risk types to the development of AGWs would also lead to a partial protective effect of 2vHPV vaccination against AGWs. Another possible explanation is that 2vHPV vaccination provides cross-protection against HPV-6/11, which was observed in the largest RCT of the 2vHPV vaccine [21]. However, only 1 of the postmarketing surveillance studies replicated this effect [33] and most could not [15, 23, 34]. Moreover, cross-protection against HPV-6/11 seems unlikely based on the large phylogenetic distance between these low-risk types and the vaccine types HPV-16/18 [32]. However, it might be that 2vHPV vaccination could prevent development of AGWs by HPV-6/11 infections, as suggested by significantly reduced HPV-6 viral loads among 2vHPV-vaccinated women relative to unvaccinated women in the Netherlands [35]. The RCT of the 2vHPV vaccine also showed that vaccination might be more effective against clinical outcomes than against HPV infections itself; the protection against cervical intraepithelial neoplasia grade 3 was higher than what was expected based on the protection against type-specific HPV infections [22]. This effect could be related to T-cell responses, triggered by the AS04 adjuvant of the 2vHPV vaccine, that are presumably broadly cross-protective [36–38].
Our results correspond to a relevant vaccine effectiveness of 23%–40%, depending on completion of the vaccination course and age at vaccination. A protective effect against AGWs further contributes to the already very attractive cost-effectiveness profile of the 2vHPV vaccine [39]. This effect was not considered in previous health economic comparisons of the 2vHPV vaccine with the 4vHPV or 9vHPV vaccine [40]. Even so, protection against AGWs should be considered a beneficial side effect, as protection against high-risk HPV types remains the main objective in 2vHPV vaccination programs.
In conclusion, in this large population-based study using data from different registries, the incidence of AGWs was significantly reduced among 2vHPV-vaccinated compared with unvaccinated women, suggestive of a partially protective effect of 2vHPV vaccination against AGWs. Our findings merit further investigation into the possible mechanisms behind the effect. This could give more insights into the natural history of AGWs and the protective effects of HPV vaccination.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Petra Oomen (Præventis) for providing the Præventis data; all general practitioners for participating in the Nivel Primary Care Database; and Statistics Netherlands for linking the data.
Disclaimer. The funders had no role in study design, data collection and analysis, interpretation of data, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Ministry of Health, Welfare and Sport of the Netherlands.
Potential conflicts of interest. M. H. reports an unrestricted research grant from Sanofi Pasteur, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health 2010; 46:S20–6. [DOI] [PubMed] [Google Scholar]
- 2. Garland SM, Steben M, Sings HL, et al. . Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis 2009; 199:805–14. [DOI] [PubMed] [Google Scholar]
- 3. Carifi M, Napolitano D, Morandi M, Dall’Olio D. Recurrent respiratory papillomatosis: current and future perspectives. Ther Clin Risk Manag 2015; 11:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park IU, Introcaso C, Dunne EF. Human papillomavirus and genital warts: a review of the evidence for the 2015 Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 2015; 61(Suppl 8):S849–55. [DOI] [PubMed] [Google Scholar]
- 5. Forman D, de Martel C, Lacey CJ, et al. . Global burden of human papillomavirus and related diseases. Vaccine 2012; 30(Suppl 5):F12–23. [DOI] [PubMed] [Google Scholar]
- 6. Vriend HJ, Nieuwkerk PT, van der Sande MA. Impact of genital warts on emotional and sexual well-being differs by gender. Int J STD AIDS 2014; 25:949–55. [DOI] [PubMed] [Google Scholar]
- 7. Dominiak-Felden G, Cohet C, Atrux-Tallau S, Gilet H, Tristram A, Fiander A. Impact of human papillomavirus-related genital diseases on quality of life and psychosocial wellbeing: results of an observational, health-related quality of life study in the UK. BMC Public Health 2013; 13:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis 2013; 13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drolet M, Bénard É, Pérez N, Brisson M; HPV Vaccination Impact Study Group . Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019; 394:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qendri V, Schurink-Van ‘t Klooster TM, Bogaards JA, Berkhof J. Ten years of HPV vaccination in the Netherlands: current evidence and future challenges in HPV-related disease prevention. Expert Rev Vaccines 2018; 17:1093–104. [DOI] [PubMed] [Google Scholar]
- 11. Howell-Jones R, Soldan K, Wetten S, et al. . Declining genital warts in young women in England associated with HPV 16/18 vaccination: an ecological study. J Infect Dis 2013; 208:1397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sonnenberg P, Tanton C, Mesher D, et al. . Epidemiology of genital warts in the British population: implications for HPV vaccination programmes. Sex Transm Infect 2019; 95:386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petráš M, Adámková V. Impact of quadrivalent human papillomavirus vaccine in women at increased risk of genital warts burden: population-based cross-sectional survey of Czech women aged 16 to 40 years. Vaccine 2015; 33:6264–7. [DOI] [PubMed] [Google Scholar]
- 14. Navarro-Illana E, López-Lacort M, Navarro-Illana P, Vilata JJ, Diez-Domingo J. Effectiveness of HPV vaccines against genital warts in women from Valencia, Spain. Vaccine 2017; 35:3342–6. [DOI] [PubMed] [Google Scholar]
- 15. Woestenberg PJ, King AJ, van der Sande MA, et al. . Medical Microbiological Laboratories; Public Health Services . No evidence for cross-protection of the HPV-16/18 vaccine against HPV-6/11 positivity in female STI clinic visitors. J Infect 2017; 74:393–400. [DOI] [PubMed] [Google Scholar]
- 16. Van den Broek IVF, van Aar F, Van Oeffelen AAM, et al. . Sexually tranmitted infections in the Netherlands in 2015. Report No.: 2016-0027. Bilthoven, Netherlands: National Institute for Public Health and the Environment, 2016. [Google Scholar]
- 17. Boersma-van Dam ME, Weesie YM, Hek K, et al. . Zorg door de huisarts. Uit: Zorg door de huisarts—Nivel Zorgregistraties Eerste Lijn: Jaarcijfers 2017 en trendcijfers 2011–2017. Verantwoording cijfers huisartsenzorg [in Dutch].. Available at: www.nivel.nl/nl/nivel-zorgregistraties-eerste-lijn/huisartsen. Accessed 25 October 2019. [Google Scholar]
- 18. van Lier A, Oomen P, de Hoogh P, et al. . Præventis, the immunisation register of the Netherlands: a tool to evaluate the National Immunisation Programme. Euro Surveill 2012; 17. [DOI] [PubMed] [Google Scholar]
- 19. Woestenberg PJ, van Oeffelen AA, Stirbu-Wagner I, van Benthem BH, van Bergen JE, van den Broek IV. Comparison of STI-related consultations among ethnic groups in the Netherlands: an epidemiologic study using electronic records from general practices. BMC Fam Pract 2015; 16:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szarewski A, Poppe WA, Skinner SR, et al. . HPV PATRICIA Study Group . Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15-25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer 2012; 131:106–16. [DOI] [PubMed] [Google Scholar]
- 21. Szarewski A, Skinner SR, Garland SM, et al. . Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk HPV types (PATRICIA randomized trial): an unexpected observation. J Infect Dis 2013; 208:1391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehtinen M, Paavonen J, Wheeler CM, et al. . HPV PATRICIA Study Group . Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89–99. [DOI] [PubMed] [Google Scholar]
- 23. Donken R, King AJ, Bogaards JA, Woestenberg PJ, Meijer CJLM, de Melker HE. High effectiveness of the bivalent human papillomavirus (HPV) vaccine against incident and persistent HPV infections up to 6 years after vaccination in young Dutch women. J Infect Dis 2018; 217:1579–89. [DOI] [PubMed] [Google Scholar]
- 24. Markowitz LE, Drolet M, Perez N, Jit M, Brisson M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine 2018; 36:4806–15. [DOI] [PubMed] [Google Scholar]
- 25. Hasanzadeh M, Rejali M, Mehramiz M, et al. . The interaction of high and low-risk human papillomavirus genotypes increases the risk of developing genital warts: a population-based cohort study. J Cell Biochem 2019; 120:12870–4. [DOI] [PubMed] [Google Scholar]
- 26. Aubin F, Prétet JL, Jacquard AC, et al. . EDiTH Study Group . Human papillomavirus genotype distribution in external acuminata condylomata: a large French national study (EDiTH IV). Clin Infect Dis 2008; 47:610–5. [DOI] [PubMed] [Google Scholar]
- 27. Sturegård E, Johansson H, Ekström J, et al. . Human papillomavirus typing in reporting of condyloma. Sex Transm Dis 2013; 40:123–9. [DOI] [PubMed] [Google Scholar]
- 28. Vandepapeliere P, Barrasso R, Meijer CJ, et al. . Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis 2005; 192:2099–107. [DOI] [PubMed] [Google Scholar]
- 29. Zhu C, Wang Y, Mao W, Zhang H, Ma J. Prevalence and distribution of HPV types in genital warts in Xi’an, China: a prospective study. BMJ Open 2019; 9:e023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skinner SR, Apter D, De Carvalho N, et al. . Human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for the prevention of cervical cancer and HPV-related diseases. Expert Rev Vaccines 2016; 15:367–87. [DOI] [PubMed] [Google Scholar]
- 31. Woestenberg PJ, King AJ, van Benthem BHB, et al. . Medical Microbiological Laboratories and the Public Health Services . Bivalent vaccine effectiveness against type-specific HPV positivity: evidence for cross-protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis 2018; 217:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bogaards JA, van der Weele P, Woestenberg PJ, van Benthem BHB, King AJ. Bivalent human papillomavirus (HPV) vaccine effectiveness correlates with phylogenetic distance from HPV vaccine types 16 and 18. J Infect Dis 2019; 220:1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Latsuzbaia A, Arbyn M, Tapp J, et al. . Effectiveness of bivalent and quadrivalent human papillomavirus vaccination in Luxembourg. Cancer Epidemiol 2019; 63:101593. [DOI] [PubMed] [Google Scholar]
- 34. Cameron RL, Kavanagh K, Pan J, et al. . Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg Infect Dis 2016; 22:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Weele P, Breeuwsma M, Donken R, et al. . Effect of the bivalent HPV vaccine on viral load of vaccine and non-vaccine HPV types in incident clearing and persistent infections in young Dutch females. PLoS One 2019; 14:e0212927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinto LA, Viscidi R, Harro CD, et al. . Cellular immune responses to HPV-18, -31, and -53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology 2006; 353:451–62. [DOI] [PubMed] [Google Scholar]
- 37. Evans TG, Bonnez W, Rose RC, et al. . A phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J Infect Dis 2001; 183:1485–93. [DOI] [PubMed] [Google Scholar]
- 38. Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin Immunol 2018; 39: 14–21. [DOI] [PubMed] [Google Scholar]
- 39. Qendri V, Bogaards JA, Berkhof J. Health and economic impact of a tender-based, sex-neutral human papillomavirus 16/18 vaccination program in the Netherlands. J Infect Dis 2017; 216:210–9. [DOI] [PubMed] [Google Scholar]
- 40. Jit M, Chapman R, Hughes O, Choi YH. Comparing bivalent and quadrivalent human papillomavirus vaccines: economic evaluation based on transmission model. BMJ 2011; 343:d5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.