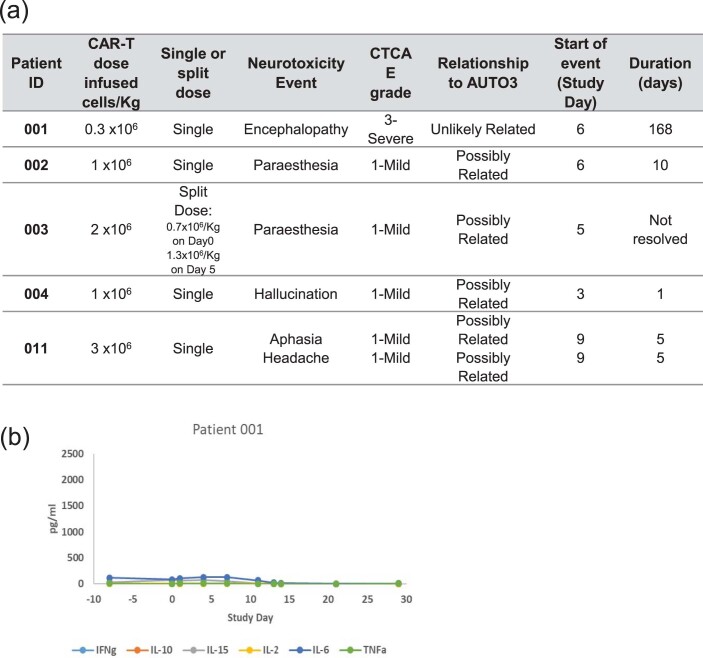
Extended Data Fig. 7. Neurotoxicity.
a) Summary of neurotoxicity events in the 5 patients who had any reported neurotoxicity AE. Note: Patient 3 had a not resolved event because the event was still ongoing when patient died due to disease progression 2.3 months after AUT03 infusion. b) Serum cytokine levels in patient 001 who experienced encephalopathy 5 days after a 0.3×106cell/kg dose of AUTO3 infusion, the encephalopathy was considered likely related to the prior infusion of intrathecal methotrexate and unlikely related to AUTO3.