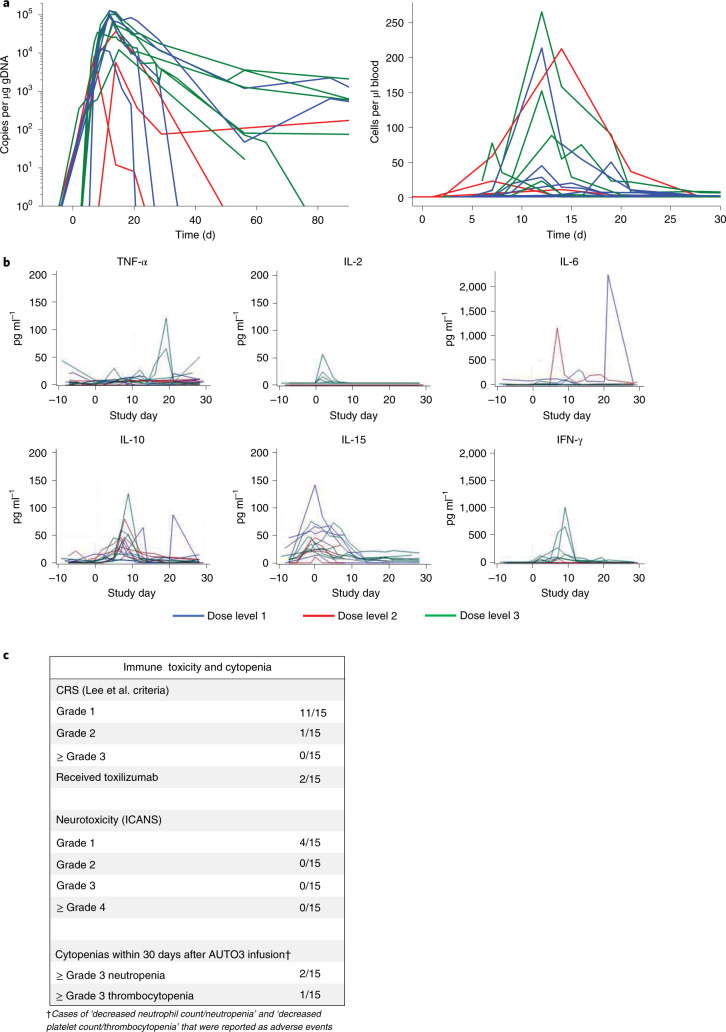
Fig. 4. Pharmacodynamics, serum cytokines and adverse events.
a, CAR T cell marking for all patients with evaluable peripheral blood samples. Copies per μg genomic DNA as detected using real-time PCR (left) in the first 90 d after infusion, and marking by flow cytometry (right) using an anti-CD19 CAR anti-idiotype as CAR T cells per μl blood in the first 30 d after infusion. b, Serum cytokines for all patients in the first 30 d after CAR T cell infusion. c, Immune toxicity and reported adverse events of cytopenias within 30 d after AUTO3 infusion.