Figure 5. BTK and IDO in human monocyte-derived DCs inhibit an inflammatory differentiation pathway driven by GATOR2 and mTORC1.
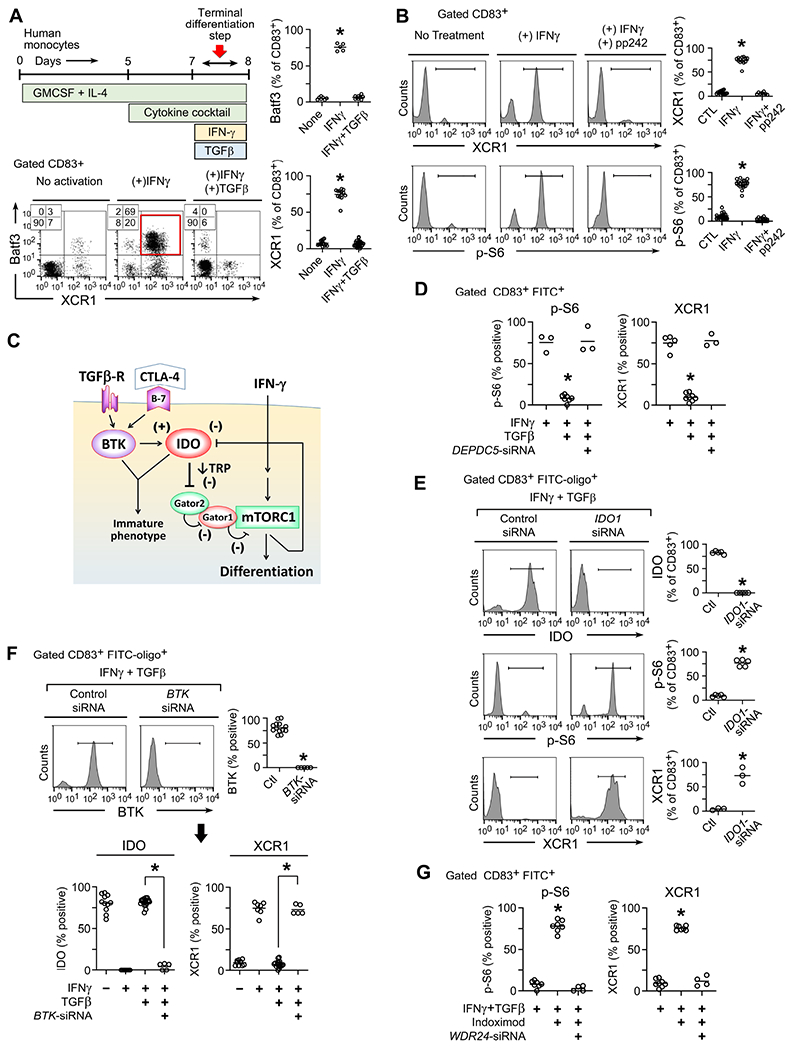
Human monocytes were cultured with growth factors and cytokine cocktail as described in Methods, followed by activation with IFNγ to drive terminal differentiation, with or without inhibitory TGFβ.
(A) In vitro culture schema, and flow cytometry analysis of gated CD83+ cells on day 8 for each treatment group. Representative of at lease 5 independent experiments. *p<0.001 by ANOVA.
(B) Analysis of DC maturation (XCR1 marker) and S6 phosphorylation by flow cytometry in cells treated with IFNγ ± mTOR-inhibitor pp242. At least 6 independent experiments per group.
(C) Proposed signaling model.
(D-G) Silencing of IDO1, BTK or components of the GATOR1-GATOR2 complex using siRNA (or scrambled siRNA control). siRNA was added day 5 along with FITC-labeled tracer oligos to identify transfected cells. Cells were analyzed by flow cytometry on day 8, and transfected cells gated as CD83+ FITC+ .
(D) Effect of silencing of the DEPDC5 subunit of GATOR1. At least 3-5 experiments per group, *p<0.001 vs both other groups.
(E) Effect of silencing IDO1. At least 3-5 experiments per group, *p<0.001 vs both other groups.
(F) Effect of silencing BTK on the stability of IDO. At least 5 experiments per group, *p<0.001.
(G) Effect of disrupting the WDR24 subunit of GATOR2 on the ability of indoximod (200 uM) to restore mTORC1 activity (p-S6) in vitro. At least 4 experiments per group, *p<0.001 vs both other groups.
See also Figure S5
