Figure 3. FKBP51 signaling via nuclear receptors to control lipid metabolism in adipose.
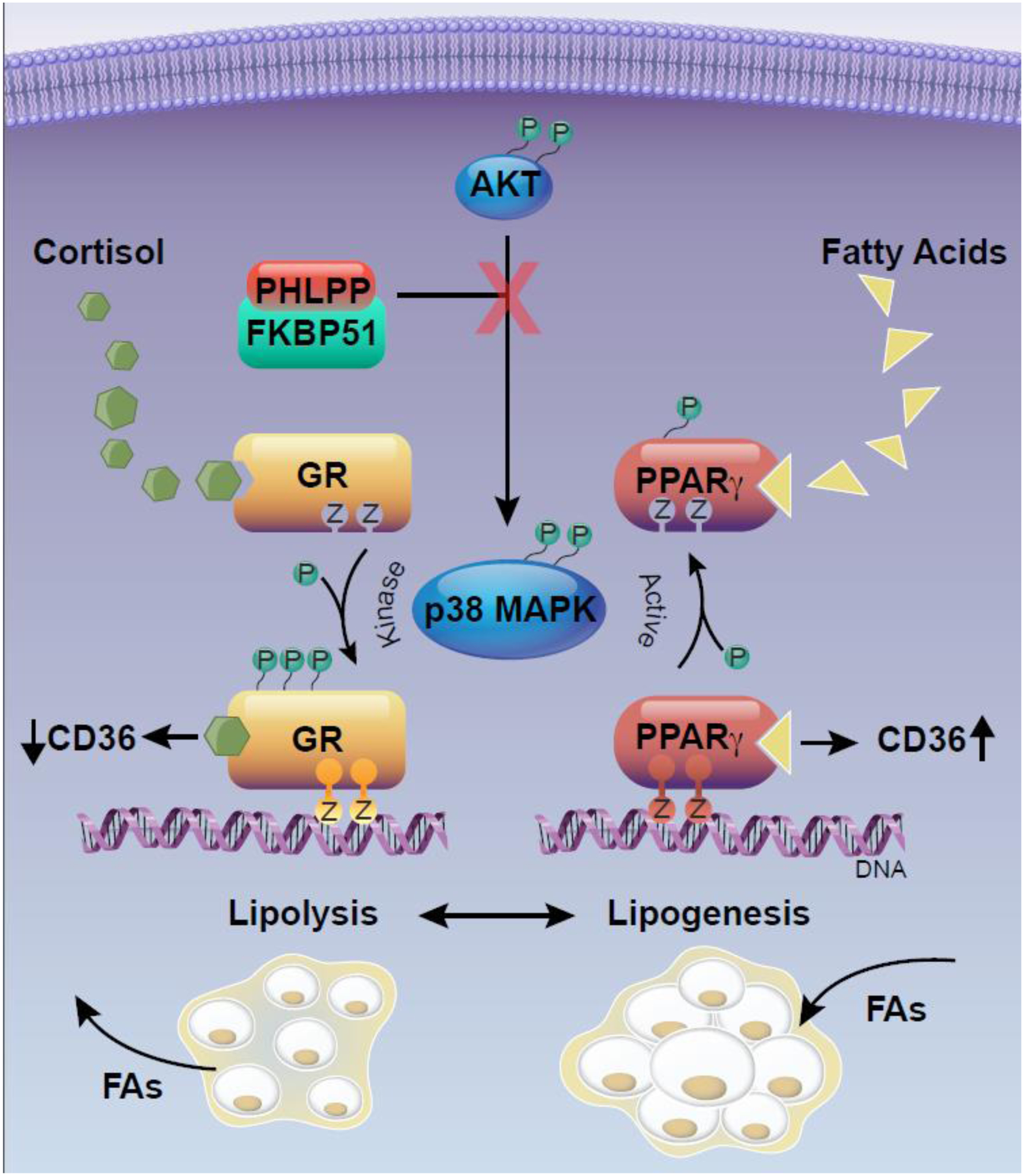
FKBP51 interaction with PHLPP suppresses AKT phosphorylation preventing its kinase activity to phosphorylate p38. The FKBP51-PHLPP-AKT interaction indirectly reduces phosphorylation mediated by p38 on the glucocorticoid receptor (GR), inhibiting cortisol-induced gene activity. In turn, FKBP51-PHLPP-AKT reduces phosphorylation of PPARγ to stimulate fatty acid-induced transcriptional activity and induction of genes for adipogenesis and lipogenesis, such as the fatty acid importer, cluster of differentiation 36 (CD36). The graphical illustration was developed by Matthew Hazzard at the University of Kentucky College of Medicine.
