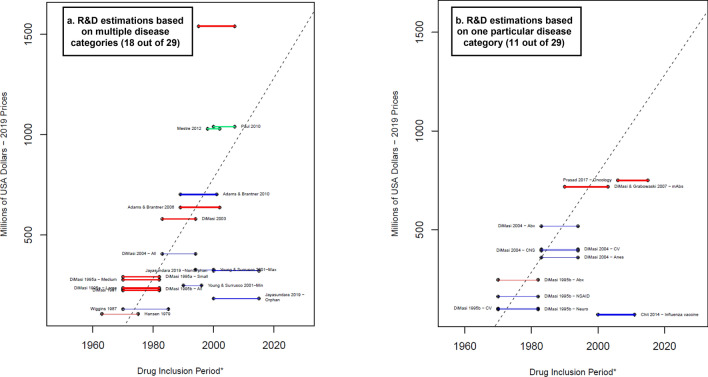
Fig. 3.
Average cash spent on R&D estimated per successful drug (considering failures)—total. Blue lines R&D costs estimation for the clinical phases. Red lines: R&D costs estimation that include an estimation for the discovery and preclinical phases. Green lines: R&D costs estimations that include an estimation for the discovery and preclinical phases as well as the R&D during the period of submission for market approval. * A thicker line represents a higher value in the suitability score, thus higher suitability of the R&D cost estimation. The length of the lines corresponds to the drug inclusion period. This is the time period considered by the authors for the selection of the drug sample. In most articles, it is the period in which the drug was first tested in humans. Nevertheless, some authors applied different definitions. For more details, see electronic supplementary information 2. Dashed line: OLS regression (excluding Jayasundara et al. [40], Chit et al. [45], and Wouters et al. [19]—oncology): . Year corresponds to the middle point of the drug inclusion period, additional details in electronic supplementary information 6. Abx anti-infectives, All the estimation includes all the observations in the sample, Anes analgesics/anesthetic, CNS central nervous system, CV cardiovascular, At&Me metabolism and endocrinology, Large large enterprises, mAbs monoclonal antibodies, Max maximum reported value, Medium medium enterprises, Min minimum reported value, Neuro neuropharmacological, NSAID nonsteroidal anti-inflammatory drugs, Small small enterprises, TB tuberculosis. Note: (1) Each line corresponds to one main R&D estimate. When more than one R&D cost estimate is reported, we refer to each by including the reference of the corresponding article and a keyword that describes the main characteristic of the R&D cost estimate. Wouters et al. [19] categorized each selected data point as high, medium, or low quality, depending on the availability and consistency of reported data. ‘High quality’ corresponds only to the estimations considered high quality observations. (2) Young and Surrusco [24] methodology included the period of submission (R&D spending over 7-years and drugs approved during the preceding 7-years). However, it did not consider the discovery and preclinical phases; therefore, it is presented as a blue line. (3) DiMasi and Grabowski [53] also considered therapeutic recombinant proteins. Source: Authors’ elaboration