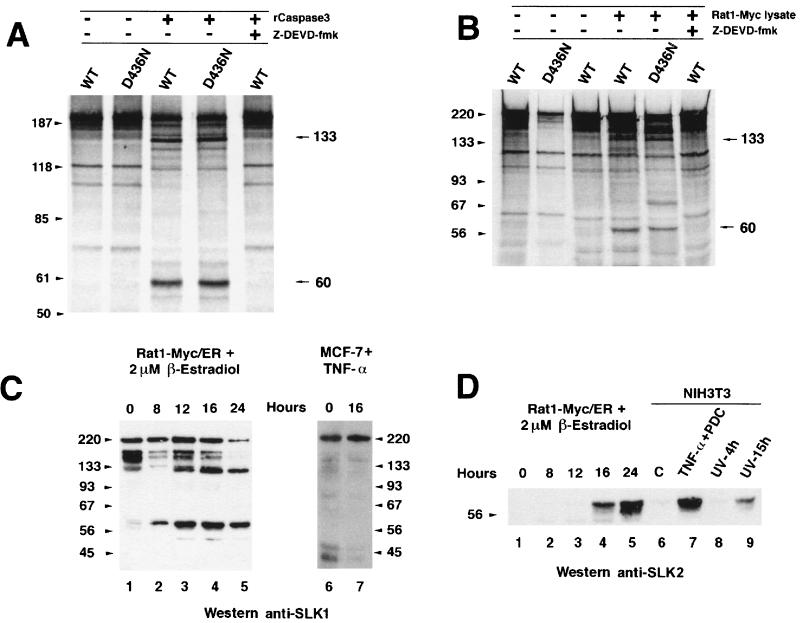
FIG. 6.
Caspase 3 cleavage of in vitro-translated wild-type (WT) and caspase 3 cleavage site mutant (D436N) SLK proteins during apoptosis. (A) In vitro-translated SLK and SLKD436N were incubated in the presence of purified caspase 3 and analyzed by SDS-PAGE. Caspase 3-specific cleavage products were observed for both constructs. Sizes are indicated in kilodaltons. (B) A similar pattern of cleavage was observed in the presence of an apoptotic cell lysate from induced (2 μM β-estradiol) Rat1-Myc/ER cells. Introduction of the D436N mutation in SLK did not prevent cleavage and release of cleavage products. Addition of the caspase 3 inhibitor Z-DEVD-fmk (50 μM) to the reaction abolished cleavage. (C) Apoptosis-induced cleavage of endogenous SLK protein in stimulated Rat1-Myc/ER cells. The levels of SLK fragments of 133 and 60 kDa progressively increased during the apoptotic response, while the levels of the full-length p220 and reactive 150-kDa species were reduced. (D) N-terminal-specific antibodies identified the 60-kDa fragment as the kinase domain in induced Rat1-Myc/ER cells and NIH 3T3 cells exposed to apoptotic triggers. NIH 3T3 fibroblasts were exposed to TNF-α (50 ng/ml) plus 10 μM PDC for 16 h or UV-irradiated (180 J/m2) and allowed to recover in growth medium for 4 and 15 h. Release of the SLK kinase domain was clearly evident. No reactivity to the 133-kDa fragment was observed, suggesting that it bears a C-terminal portion of SLK.