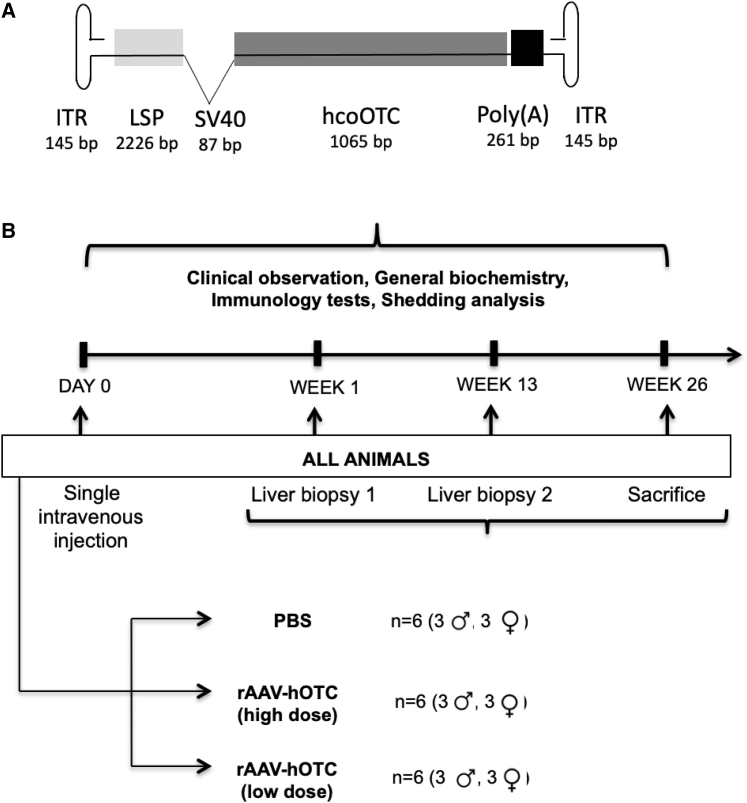
Figure 1.
Experimental design for preclinical assessment of AAVLK03.LSP.hOTC
(A) Vector schematic of AAVLK03.hOTC. The liver-specific promoter (LSP) contains an ornithine transcarbamylase (OTC) enhancer and the human α1 anti-trypsin promoter. (B) Experimental design of the toxicity study. Three investigational groups of juvenile cynomolgus macaques were monitored for 26 weeks after a single intravenous injection of the AAVLK03.hOTC vector before sacrifice and organ collection. Over a 26-week monitoring period, biofluids were collected at regular intervals, and needle liver biopsies were performed at 1 and 13 weeks post-vector administration. hcoOTC, human codon-optimized OTC transgene; ITR, inverted terminal repeat; PBS, phosphate-buffered saline; rAAV, recombinant adeno-associated viral vector.