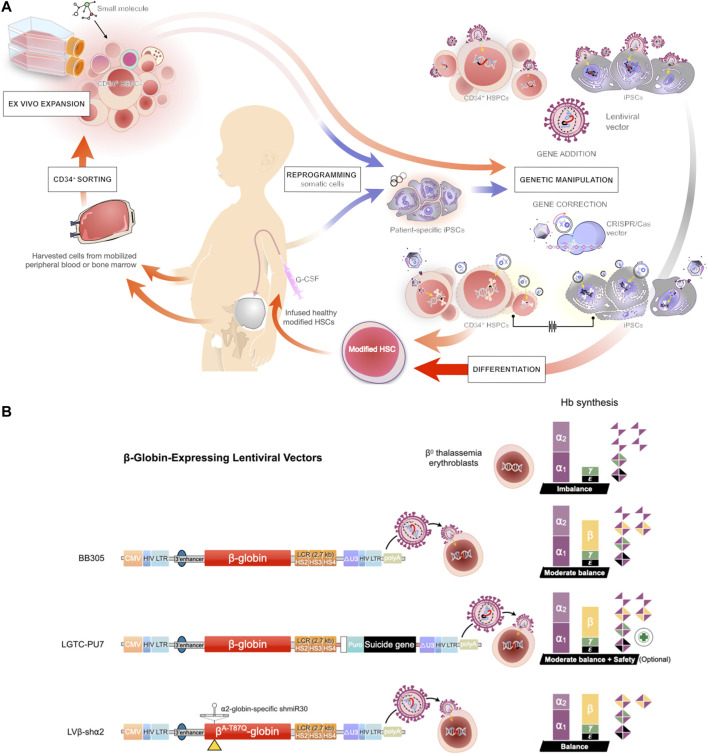
FIGURE 2.
Gene therapy approaches for transfusion-dependent β-thalassemia patients (A) Schematic representation of the protocols for gene therapy in β-thalassemia. Induced pluripotent stem cells (iPSCs) are reprogrammed from somatic cells and differentiated into HSCs, or the HSCs are directly harvested from the mobilized peripheral blood or bone marrow of a patient and further manipulated by ex vivo maintenance or expanded by co-culture with a cocktail of cytokines and small molecules. The harvested HSCs are subjected to gene transfer (addition) by lentiviral vector or gene editing using CRISPR/Cas9 technology. The engineered HSCs are then applied to replace the inherited defective β-globin gene of a patient and restore function of the erythroid lineage in β-thalassemia (B) Prospective of modified lentiviral vector expressing the β-globin gene. The three indicated lentiviral vectors encode the β-globin chain under the control of the human β-globin promoter and hypersensitive sites (HS) of the β-globin locus control region (LCR) (Top) A LentiGlobin™ BB305 vector, the current gene therapy drug product for the treatment of non-β0/β0 thalassemia (Middle) A modified LentiGlobin™ BB305 vector, LTGC-PU7, encodes the β-globin chain and the puromycin N-acetyltransferase (PAC) cassette with an optional suicide gene (Bottom) LentiGlobin™ BB305 is modified to express the miR-30-based short hairpin RNA (shRNA) selectively targeting the α2-globin mRNA, the therapeutic option for β0/β0 thalassemia patients.