FIGURE 4.
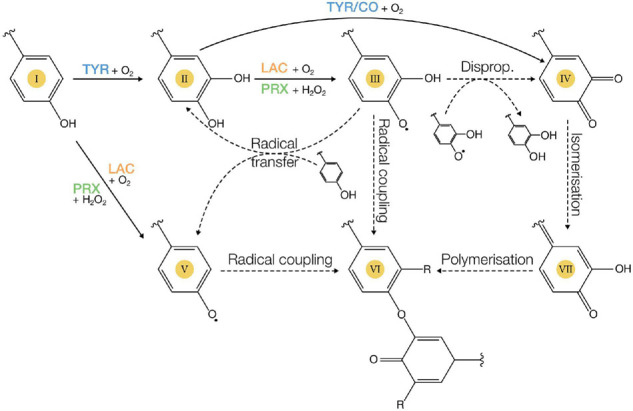
Classical reactions catalysed by PPOs (CO, catechol oxidase; TYR, tyrosinase), LACs, and class III PRXs. Enzymatic and non-enzymatic reactions are indicated with solid and dashed arrows respectively on the example of simple mono- and diphenolic molecules. Monophenol hydroxylation (I → II) is generally considered to be exclusive to TYRs. The one electron oxidation of diphenols (II) by LACs or PRXs leads to a semiquinone radical (III). This can then couple with another semiquinone to form a dimer or polymer (VI), transfer its radical to another compound (III + I → II + V), or disproportionate with another semiquinone to form a quinone (2 III → IV + II). Quinones (IV) can isomerise to quinone methides (VII), which undergo non-enzymatic coupling reactions to form dimers (VI). Lastly, LACs and PRXs can also oxidise monophenols (I) into phenoxy radicals (V).
