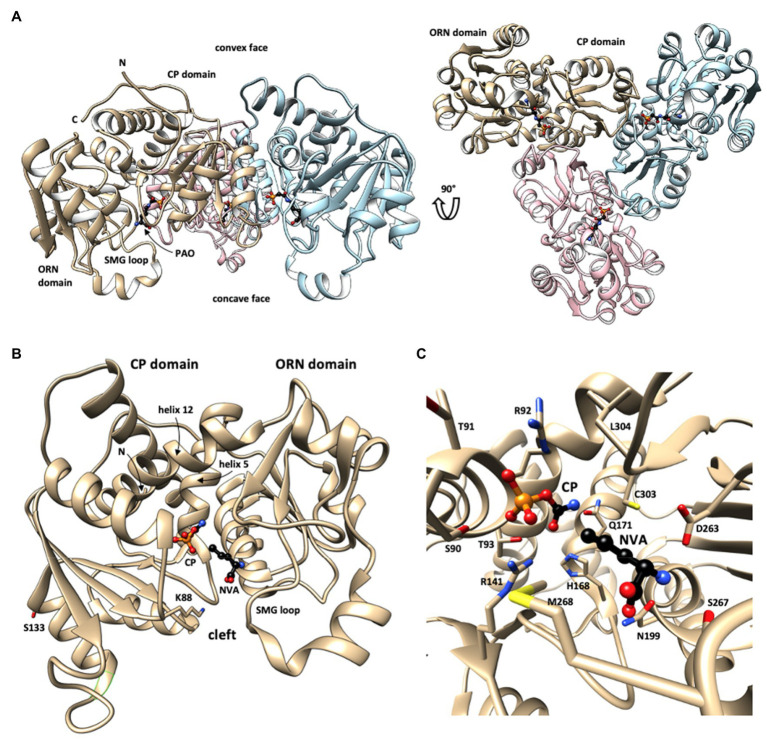
Figure 2.
Structure of human OTC. (A) Overall structure of the OTC homotrimer with bound phosphonoacetyl-L-ornithine (PAO) in sideview (left) and top view (right), revealing the 3-fold rotational symmetry (every monomer in different color; one monomer labeled). This structure shows a largely open state of OTC monomers. (B) Fold of the closed state of monomeric OTC with N-terminal (CP) and C-terminal (ORN) domains. Long helices linking the two domains (helices 5 and 12) are labeled. Substrate-binding sites in the catalytic cleft between the two domains are identified by bound CP and L-norvaline (NVA, an ORN analogue). Selected residues involved in identified secondary modifications (acetylation at lysine 88, phosphorylation at serine 123) are shown. (C) Close-up showing bound substrates and involved critical OTC residues (see text). OTC structural data were taken from PDB 1OTH (A) and 1PDB C9Y (B,C). The structures are given in backbone representation with individual sidechains visualized in stick representation and substrates shown as ball-and-stick models. N,C: N- and C-termini. Figure prepared with UCSF Chimera v1.11.2.