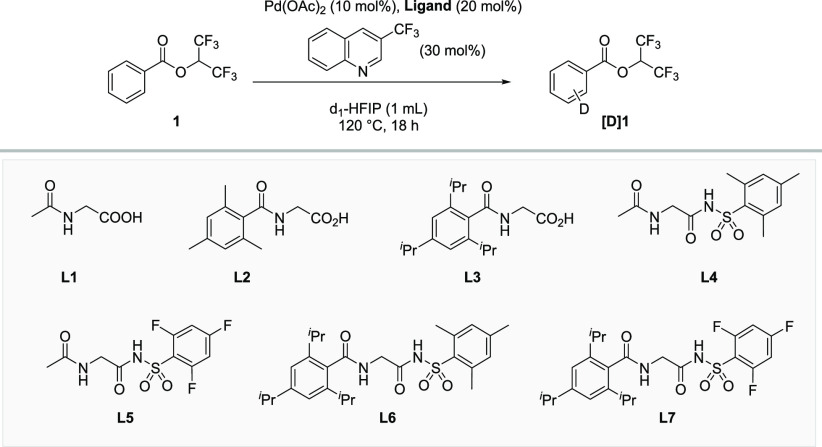
Table 1. Optimization of the Ligand Structurea,b.
| D content
(%) |
||||||
|---|---|---|---|---|---|---|
| entry | ligand | yield (%) | ortho | meta | para | total D content |
| 1 | L1 | 99 | 11 | 50 | 23 | 1.66 |
| 2 | L2 | 95 | 22 | 73 | 41 | 2.42 |
| 3 | L3 | 97 | 24.5 | 79 | 47 | 2.65 |
| 4 | L4 | 98 | 4 | 46 | 23 | 1.27 |
| 5 | L5 | 98 | 7 | 35 | 21 | 1.05 |
| 6 | L6 | 97 | 5 | 72 | 46 | 2.08 |
| 7 | L7 | 97 | 17 | 90 | 74 | 2.87 |
| 8c | L7 | 95 | 39 | 95 | 84 | 3.51 |
| 9c,d | L7 | 99 | 34 | 60 | 32 | 2.15 |
| 10c | no L7 | 98 | 0 | 0 | 0 | 0 |
| 11c,e | L7 | 94 | 62 | 95 | 95 | 4.05 |
Reactions were performed on a 0.1 mmol scale.
Yields and degrees of deuteration were determined by 1H NMR spectroscopy using mesitylene as an internal standard. The total deuterium content was determined by mass spectrometry.
The reaction was performed with D2O/HFIP (7:3) as the solvent. Since D2O is used as part of the solvent system, this corresponds to an excess of approximately 390 equiv.
No 3-trifluoromethylquinoline.
The reaction was performed with a reaction time of 48 h.