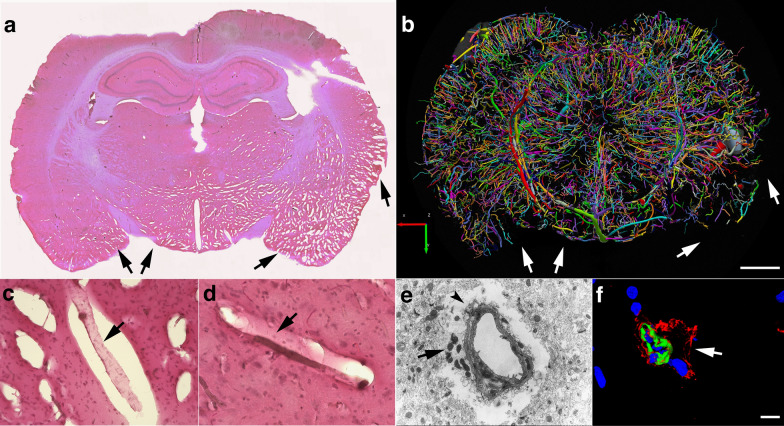
Fig. 8.
Chronic enlargement of paravascular spaces in the brain at 13 months post-blast exposure. Coronal sections from a blast-exposed animal were stained with hematoxylin and eosin a, c, d. The general pathology showing enlarged paravascular spaces is shown (arrows in a). In this animal, the histopathology is mostly concentrated in one hemisphere and involves the amygdala, insula, piriform cortex, ventral thalamus and hypothalamus. In the contralateral hemisphere, pathological changes are limited to the piriform cortex and hypothalamus. Panel b shows the reconstructed cerebral vasculature from a 3.75 mm micro-CT optical section that includes the region shown in a. The vascular reconstruction b mirrors the enlarged paravascular spaces seen in panel a where the hypothalamus, ventral thalamus and, most strikingly, the amygdala and piriform cortex show reduced perfused vessels (arrows) and enlarged paravascular spaces. The lack of vascular tracing (arrows in a, b) suggests hypoperfusion of the corresponding areas and could be a consequence of perivascular astrocytic degeneration. Panel c shows a detached artery (arrow) within an enlarged paravascular space in the ventromedial hypothalamus. Panel d shows a constricted artery (arrow) in the ventral posteromedial nucleus from which the adventia has been detached. Panel e shows an electron micrograph of a cortical arteriole barely attached to the parenchyma through a few degenerating astrocytic endfeet (arrow). Arrowhead in e shows enlarged paravascular space. Panel f shows immunohistochemical analyses of a hypothalamic arteriole with a disrupted smooth muscle layer (α-SMA+, green) and detached adventitia (col IV+, red); DAPI, blue. Scale bars, 2 mm a, b; 40 µm c, d; 1.5 µm e; 15 µm f