Table 1.
Optimization of reaction conditions.[a]
|
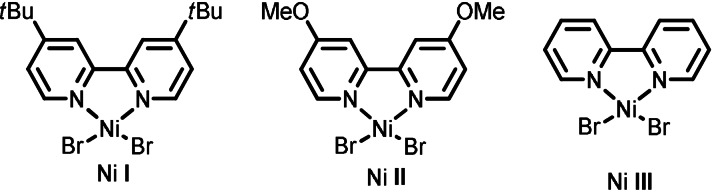
Entry |
Variation from conditions |
Yield [%][b] |
|---|---|---|
|
1 |
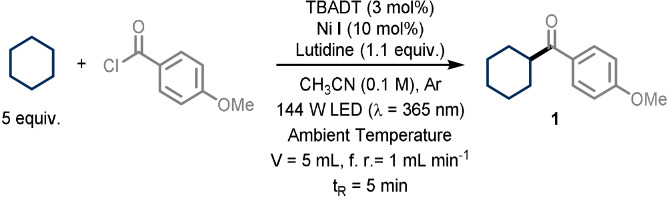
None |
68 (65) |
|
2 |
TBADT (1 mol %) |
50 |
|
3 |
Ni I (5 mol %) |
53 |
|
4 |
Ni II |
39 |
|
5[c] |
Ni III |
28 |
|
6 |
36 W |
52 |
|
7 |
DBU or Pyridine |
– |
|
8 |
2.5 equiv of cyclohexane |
41 |
|
9 |
10 equiv of cyclohexane |
70 |
|
10[d] |
Batch conditions |
50 |
|
11 |
Absence of TBADT, Ni or Light |
– |
|
12 |
Scale up, 5 mmol |
55 |
|
| ||
|
| ||
[a] Cyclohexane (5 equiv), 4‐methoxy benzoyl chloride (0.5 mmol), in CH3CN (5 mL). [b] Yields determined by 1H NMR spectroscopy using trichloroethylene as external standard, yield of the isolated product is reported in brackets. [c] The reaction mixture is heterogeneous, therefore it was conducted under batch conditions, please see the Supporting Information for further information. [d] The reaction mixture was irradiated for 12 hours, please see the Supporting Information for further information.