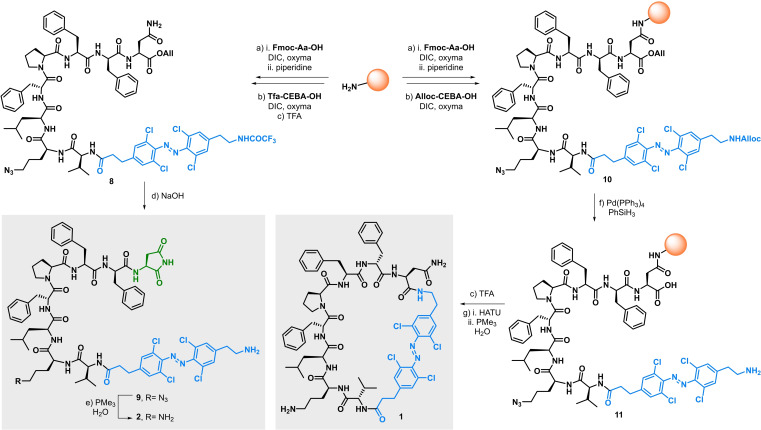
Scheme 2.
Synthesis of linear and cyclic Visible Light photoswitchable tyrocidine A analogues 1 and 2. Reaction conditions: a) see Supporting Information for details on manual and microwave‐assisted SPPS methods. b) Alloc‐CEBA‐OH or Tfa‐CEBA‐OH (2.3 equiv), DIC (2.5 equiv), oxyma (2.5 equiv), DMF, rt, 16 h. c) TFA/CH2Cl2 (95 : 5), rt, 5×45 min. d) NaOH (3.5 equiv), THF, MeOH, H2O, rt. e) PMe3 (3 equiv), H2O, THF, 0 °C to rt. f) Pd(PPh3)4 (10 mol %), PhSiH3 (20 equiv), CH2Cl2, rt, 15 min. g) HATU (2 equiv), DIPEA (6 equiv), DMF, rt, 16 h; then PMe3 (5 equiv), H2O, 0 °C to rt. Alloc=Allyloxycarbonyl; DIC=N,N’‐diisopropylcarbodiimide; oxyma=ethyl cyanohydroxyiminoacetate; DMF=dimethylformamide; TFA=trifluoroacetic acid; THF=tetrahydrofuran HATU=1‐[bis(dimethylamino)methylene]‐1H‐1,2,3‐triazolo[4,5‐b]pyridinium 3‐oxide hexafluorophosphate; DIPEA=diisopropylethylamine.