Abstract
The skin is home to a community of skin microbiota including bacteria, viruses and fungi, which are widely accepted to be of importance for skin homeostasis but also associated with skin diseases. Detailed knowledge on the skin microbiota composition and its changes in a number of skin diseases is available. Yet, specific interactions between microbes and the host skin cells or how they communicate with each other are less well understood. To identify, understand and eventually therapeutically exploit causal relationships of microbial dysbiosis with disease, studies are required that address the receptors and mediators involved in host‐microbe interactions. In this perspective article, we provide an outlook on one of such receptors, namely the aryl hydrocarbon receptor (AHR). The AHR is well known for being a ligand‐activated transcription factor regulating the proliferation, differentiation and function of many cell types present in the skin. Its targeting by anti‐inflammatory therapeutics such as coal tar and Tapinarof is effective in atopic dermatitis and psoriasis. AHR signalling is activated upon binding of wide variety of small chemicals or ligands, including microbiota‐derived metabolites. New evidence has emerged pointing towards a key role for epidermal AHR signalling through skin microbiota‐derived metabolites. In response, AHR‐driven expression of antimicrobial peptides and stratum corneum formation may alter the skin microbiota composition. This a self‐perpetuating feedback loop calls for novel therapeutic intervention strategies for which we herein discuss the requirements in future mechanistic studies.
Keywords: antimicrobial peptides, aryl hydrocarbon receptor, epidermal differentiation, inflammatory skin diseases, microbiome, nuclear receptor
1. INTRODUCTION
The skin is continuously challenged by the outside environment and harbours multiple receptors that aid in the communication with the environment and contribute to host defence. The aryl hydrocarbon receptor (AHR) has for many years after its discovery primarily been considered a receptor for xenobiotic pollutants, whose activation results in xenobiotic metabolism and elimination, but also taking part in cellular and tissue toxicity. 1 The principle of AHR activation is well known. In brief, small molecular weight chemicals non‐covalently bind to a pocket in the AHR protein, resulting in the release of chaperoning proteins, translocation to the nucleus, dimerization with its partner molecule, aryl hydrocarbon receptor nuclear translocator (ARNT) and ultimately the transcription of target genes. This basic signalling mechanism is fine‐tuned by a plethora of cell‐specific factors, such as competition for transcription co‐factors, possibilities to interact with other signalling pathways such as NFkB 2 and target gene accessibility. 3 Importantly, the role of ligand characteristics (eg affinity, degradability and chemistry) for the dynamics of the signalling 4 outcome needs further research.
Importantly, many natural and physiological AHR ligands and their sources were identified over the years (reviewed by Murray and Perdew 5 ). Key physiological events are mediated or co‐regulated by AHR signalling, which in the in skin encompass cellular proliferation, 6 differentiation, 7 , 8 , 9 maturation 10 , 11 and migration. 12 Here, we focus on the evidence for AHR activation by skin microbiota as well as potential consequences of this activation for both host and microbiota related to skin health and disease.
2. HOST AHR SIGNALLING VIA SKIN MICROBIOTA
AHR ligands produced by skin microbiota may permeate through the stratum corneum, epidermis and skin appendages such as hair follicles, sweat and sebum glands, which serve as a port d’entree for skin microbiota to colonize the deeper layers of the skin. Most well known and studied microbial metabolites that modulate AHR through direct ligand binding are tryptophan metabolites, like indoles and kynurenines, mostly identified from (murine) gut microbiome studies. 13 The AHR activating potential by tryptophan metabolites is complex as weak agonist (like indole) in the presence of a potent agonist will exhibit antagonist activity. 14 However, the differences in ligand affinity between mouse and human AHR should be taken into consideration here as indole exhibits greater agonist activity towards the human AHR compared to the mouse AHR. 15 Short‐chain fatty acids (SCFA) produced by microbes, like butyrate, can enhance AHR activation indirectly when AHR ligands are present. 16 , 17 , 18 Recently, evidence for quorum sensing by AHR was demonstrated for Pseudomonas aeruginosa whereby the AHR controls infection dynamics and orchestrates host defence. 19 Most studies on the AHR‐microbiome axis focus on the gut, where the AHR has been firmly established as a key receptor for gut microbial metabolites and regulator of organ homeostasis through modulating intestinal immunity, 20 enhancing epithelial barrier functioning 21 and inducing xenobiotic metabolism, 22 a topic recently reviewed by Dong and Perdew. 23 Besides local tissue‐specific AHR targeting via microbial metabolites, systemic entry of metabolites formed at barrier organs, resulting in cross‐compartment effects of gut microbiota on many organs (eg gut‐brain axis, 24 gut‐lung axis, 25 gut‐skin axis 26 ), has far‐reaching implications for understanding mechanisms of disease, and for the development of disease therapeutics and prevention strategies. Similar effects could hold true for skin‐derived AHR ligands which may enter the bloodstream, although direct evidence is not yet available.
All barrier organs, and, in particular, the skin are continuously challenged by the exposome (or the collection of all environmental factors impacting human health) which also impacts the microbiome in its composition and metabolism, exemplified by dietary intake, 27 environmental pollution 28 , 29 and UV exposure. 30 , 31 Epidemiological and experimental studies on exposome‐associated AHR ligands (eg certain pollutants and dietary compounds, or UV radiation‐generated endogenous tryptophan metabolites) and signalling thus need to consider the additional influence of exposome‐mediated alterations in the microbiome and changes in microbial‐derived AHR ligands thereof.
Studies addressing the AHR activating potential and downstream effects by skin microbiota are mostly performed by exposure of AHR reporter cells or primary skin cells to whole bacteria, bacterial lysates or purified metabolites, 32 , 33 , 34 , 35 after which known AHR ligands with differences in receptor affinity and turnover times, like TCDD or FICZ are used to substantiate findings. 36 Skin microbiota include a substantial fungal community, which is dominated by Malassezia species. 37 Malassezia produce a variety of indole metabolites having AHR activating potential (eg indirubin, Malassezin, FICZ). 35 Although Malassezia dysbiosis is associated with skin diseases (eg seborrheic/atopic dermatitis), 38 , 39 in dept studies on the effects of the metabolites formed by the different Malassezia species in skin or co‐cultures of Malassezia isolates with (organotypic) skin are scarce. 40 Evidence from studies on skin commensal bacteria indicate that AHR activation after skin cell exposure to commensal bacteria (eg S. epidermidis, S. warneri and C. aurimucosum 32 , 36 ) or specific microbial metabolites (eg indole‐3‐aldehyde 34 ) contributes to epidermal skin barrier function, 36 , 41 negatively regulates immune cell responses 10 and dampens (experimentally induced) skin inflammation. 34 These effects are generally seen also for non–microbial‐derived AHR ligands that are able to activate the AHR which provide a rationale to the therapeutic targeting of AHR for two major inflammatory skin diseases, atopic dermatitis and psoriasis 42 , 43 , 44 both characterized by skin barrier defects and chronic inflammation. Furthermore, AHR activation upregulates antimicrobial peptides expression in keratinocytes, potentially altering skin microbiota composition and restoring dysbiosis in atopic dermatitis. 45 Recently, also for hidradenitis suppurativa, another severe inflammatory skin condition, AHR therapeutic targeting was proposed. Changes in the skin microbiota are characteristic for the disease, with fewer bacteria present known to produce beneficial AHR ligands from tryptophan suggesting a loss of AHR‐mediated host‐microbe communication through altered tryptophan catabolism. 33 Altered tryptophan metabolism is a feature also associated with dysbiosis in atopic dermatitis. 46 , 47 Hence, altered levels or composition of microbial‐derived AHR ligands may contribute to disease pathophysiology. In other words, due to dysbiosis, ligand availability changes, which normally drive constitutive AHR signalling required for epidermal structural integrity and barrier function, as wells as skin immunity. This concept was recently underscored by the finding that high levels of CYP1A1 enzymatic activity lead to exaggerated skin inflammation and phenocopy AHR deficient mice. 48 The authors proposed that CYP1A1 may deplete the AHR natural ligand pool in the skin (eg microbial metabolites or the UV‐irradiation generated molecule FICZ)) by phase I metabolism and thereby release the break that AHR normally holds on skin inflammation. Unfortunately, metabolomic analysis to determine actual metabolite levels, composition and source were lacking and remain to be further explored. The need for active AHR signalling as protection against overt skin inflammation is further indicated by the vemurafenib‐induced inflammatory skin rashes in melanoma patients. Vemurafenib was found to act as an AHR antagonist and elevated pro‐inflammatory molecules in keratinocytes in vitro. 49
3. MECHANISTIC INSIGHTS AND THE CHALLENGES OF EXPERIMENTAL APPROACHES
Overall, mechanistic insights into the molecular signalling events that drive AHR‐mediated effects in skin are still under‐explored. This is of particular interest given the promiscuity of the AHR towards various ligands and its canonical (through dimerization with ARNT) and non‐canonical signalling pathways, as mentioned before. Up or downstream interaction of AHR with signalling pathways important for skin structure and function demonstrate the need for in depth molecular analysis to pinpoint AHR’s mode of action in host‐microbe interactions. Herein, the setup of studies is a crucial factor in providing key evidence for microbiota‐driven AHR activation contributing to skin homeostasis. The interspecies differences in receptor affinity known for the murine and human AHR 16 , 50 should be taken into consideration when extrapolating study findings from mouse to human. Alternatively, humanized AHR mice or low affinity (human) variant (Ahrd / d) 51 may better predict AHR activation from host‐microbe interactions and its consequences for human health and disease. In addition, human organotypic skin models for microbiome research emerge as alternatives to mouse models. 52 Studies using purified metabolites at artificial concentrations and specific time of dosage indeed provide proof‐of‐concept on the potential for AHR activation. However, the resulting effects may not reflect the actual mode of action as metabolite concentration, turnover, bioavailability and interaction with other microbial metabolites and host proteins are not captured. In addition, studies on single bacterial (laboratory) strains or mixtures thereof may be less representative as bacterial transcriptomes are influenced by the host environment and tissue state. Indeed, individual clinical isolates may differ, and reflect interpersonal differences regarding the production of metabolites capable of AHR activation. 53
To date, only few studies exist addressing AHR signalling in skin investigated patient cohorts 33 , 34 , 48 from a microbiome perspective. Because these had only small sample numbers (<20 per group), there is a need for integrated multi‐omics approaches to investigate and correlate microbiome composition (16S rRNA gene sequencing or ideally metagenomics) with microbial metabolome and host transcriptome/epigenome. Given the wide expression of AHR in a multitude of cell types in skin, conventional transcriptomics studies using bulk RNA from full skin biopsies may mask potential cell type‐specific effects hence favouring single‐cell approaches. Functional analysis of in vivo host‐microbe signatures in organotypic models of human skin can provide proof‐of‐principle data to pinpoint the role of the AHR using genome editing strategies or selective AHR inhibition. The first would be preferred given potential unspecific targeting by pharmacological inhibitors that might mask the actual AHR‐mediated signalling events and its downstream cellular effects.
4. SELF‐PERPETUATING FEEDBACK LOOP FROM AHR ACTIVATION TO MICROBIOME COMPOSITION
Besides the above‐discussed function of the AHR in communicating signals from the microbiome to the host, the known downstream effects of AHR activation on the epidermis and stratum corneum formation could provide a feedback mechanism controlling the skin microbiome composition. Although ligand promiscuity of microbial metabolites may also be a factor to consider, AHR activation is involved in the transcription of genes for epidermal differentiation proteins, 8 , 54 lipid synthesis 55 , 56 and sebocyte differentiation. 57 Expression of these genes may change stratum corneum structure, lipid and amino acid content, skin surface pH and sebum levels. Of note, these are all processes, which ultimately can affect the skin microbiome composition. 58 Specifically, the induction of filaggrin may be of importance considering the key functions of this protein in generating natural moisturizing factor (NMF) by its proteolytic cleavage and deamination during keratinocyte terminal differentiation. 59 NMF is important for skin barrier function and NMF levels inversely correlate to AD disease severity. 60 Recently, adhesion of Staphylococcus aureus to the stratum corneum was found to correlate to NMF levels. 61 Low levels of NMF are found in skin of atopic dermatitis (AD) and ichthyosis vulgaris (IV) patients which harbour loss‐of‐function mutations in the filaggrin gene 60 or have downregulated filaggrin levels due to the local inflammatory milieu. 62 Skin microbiome dysbiosis in IV patients is characterized by a low abundance of specific bacteria, called gram‐positive anaerobic cocci (GPAC). 63 GPAC abundance correlated to NMF levels in a dose‐dependent manner, possibly due to their nutrient requirement for histidine, one major constituent of NMF. Also in AD, GPAC are less abundant. 46 It would be highly interesting to investigate if AHR targeting drugs in AD, like in the Tapinarof trials, restore microbial dysbiosis, whether GPAC levels return to normal, and if this coincides with normalized filaggrin expression and NMF levels in the stratum corneum. The study on the effects of coal tar treatment (and AHR activation) on the microbiome of AD patients and healthy volunteers did not detect differences in GPAC before or after treatment, albeit this study was underpowered to detect such differences and the time period of treatment may have been too short. 45 Of note, therapeutic AHR ligands like polycyclic hydrocarbons in coal tar and Tapinarof may also exert direct effects on the microbiome by their antimicrobial activity, 64 adding even more complexity to the various modes of AHR‐directed host‐microbe interplay, its involvement in disease pathophysiology and therapeutic potential (Figure 1).
FIGURE 1.
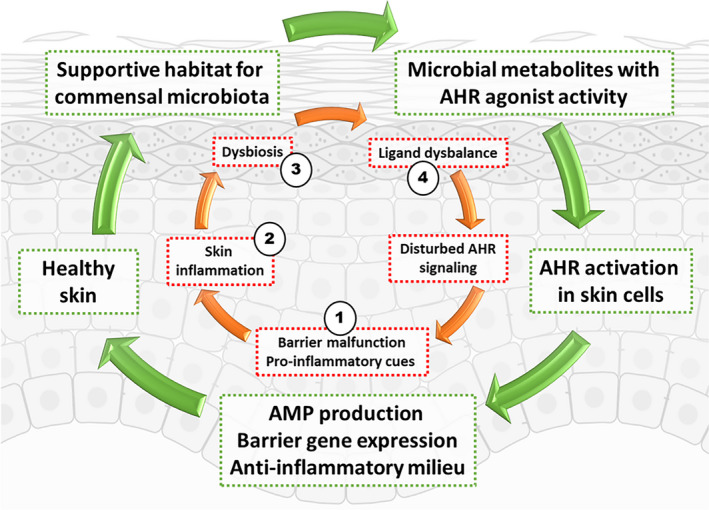
Concept of a microbiota‐AHR feedback loop that is important for the maintenance of skin homeostasis. AHR ligands derived from skin bacteria or fungi contribute to AHR signalling in the skin. AHR signalling in turn controls expression of skin barrier genes such as filaggrin and antimicrobial peptides. In addition, the AHR also plays a role in modulating immune cell function, and thus inflammatory responses to an insult. The green arrows denote the default, healthy situation. While the red arrows denote pathophysiology in inflammatory skin disease, such as atopic dermatitis or psoriasis, or where environmental pollutants such as diesel exhaust cause inflammation. Therapeutic opportunities are proposed, such as in 1) skin barrier repair, 2) anti‐inflammatory drugs, 3) pro‐pre‐antibiotics and 4) AHR ligand/antagonist supplementation or AHR signalling modulators (eg CYP1A1 inhibitors). The presence of non–microbiota‐derived AHR agonists present in skin, from other environmental sources or dietary‐derived agonists reaching the skin are not depicted here. The required level of AHR signalling to maintain skin homeostasis is unknown
Next to the potential AHR‐mediated changes on the feeding ground of skin microbiota, the innate host defence mechanisms by means of the skin AMP repertoire can be modulated by AHR signalling. 45 Major epidermal AMPs like beta‐defensins, S100 proteins and SKALP/elafin are inducible by AHR ligands, although this induction could coincide with the driving forces of AHR agonist on keratinocyte terminal differentiation. This AHR‐mediated expression of genes within the epidermal differentiation complex (EDC) locus on chromosome 1 including filaggrin, hornerin and late cornified envelope proteins 8 , 54 , 65 further aids in the antimicrobial defence. For long, these proteins were considered classical terminal differentiation proteins, involved in structural integrity of the epidermis and formation of cornified envelopes. Recent findings highlight their antimicrobial activity 66 , 67 and classifying them as so‐called cationic intrinsically disordered AMPs or CIDAMPs. 67
These studies clearly point towards a concept where the AHR provides the skin with a communication hub for bi‐directional interactions with its microbiota. Considering that the AHR is utilized as a target for intervention, mostly using specific agonists for dampening skin inflammation, we must consider the additional effects of this new drug class on the microbiome, which could feed into a self‐perpetuating feedback loop—either beneficial or like a vicious circle. Therapeutic approaches can be topically, or via the diet, exploiting the gut‐skin axis. Beyond using AHR agonists, probiotic bacteria (with known potential for AHR metabolite formation) might be useful in manipulating the microbiome‐AHR‐skin health axis. 36 Alternatively, prebiotic strategies steering the growth of skin microbes, which produce desirable AHR‐modulating metabolites may provide new therapeutic modalities for the dermatology field. Herein, it is of importance to critically address the characteristics of the induced ligand‐activated AHR signalling considering the adverse effects of uncontrolled AHR activation in the skin. 12 , 64 , 68 Finally, several lines of evidence provide a mechanistic and therapeutic rationale for specific lifestyle interventions in patients with chronic inflammatory skin conditions. These are (i) anti‐inflammatory effects of dietary intervention studies with AHR ligands, (ii) the ability to modulate AHR ligand production in the gut by dietary intake and (iii) the known systemic entry of AHR ligands produced in the gut (or via second‐pass effects in the liver) and beneficial effects on skin function.
5. CONCLUSION
The ligand‐activated transcription factor AHR is a sensor for parts of the exposome, and intrinsically important for skin health and homeostasis, but may also mediate adverse functions depending on the context, for example chronic exposure to xenobiotic ligands. Evidence emerges that microbes of the skin are a relevant source of AHR ligands, thereby ensuring that their own habitat remains stable. In inflammatory skin diseases with characteristic dysbiosis, such as atopic dermatitis or psoriasis, this balance is perturbed. Understanding that the relationship between AHR and the skin microbiota is reciprocal will help define future experiments and research. Ultimately, therapeutic approaches might seek to balance the skin microbiome and re‐balance its AHR‐beneficial signature.
CONFLICT OF INTEREST
Authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
EB conceived and wrote the manuscript. CE and GP critically reviewed and edited the manuscript. CE and EB designed the figure.
ACKNOWLEDGEMENTS
EB receives funding from The Dutch Research Council (ZonMW Off Road; 451001028; VENI 91616054), GHP is funded by National Institutes of Health Grants (ES028244). CE receives funding from the Deutsche Forschungsgemeinschaft (DFG ES103/9‐1).
van den Bogaard EH, Esser C, Perdew G. The aryl hydrocarbon receptor at the forefront of host‐microbe interactions in the skin: A perspective on current knowledge gaps and directions for future research and therapeutic applications. Exp Dermatol. 2021;30:1477–1483. 10.1111/exd.14409
REFERENCES
- 1. Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vogel CF, Khan EM, Leung PS, et al. Cross‐talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor‐kappaB. J Biol Chem. 2014;289(3):1866‐1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gargaro M, Scalisi G, Manni G, Mondanelli G, Grohmann U, Fallarino F. The landscape of AhR regulators and coregulators to fine‐tune AhR functions. Int J Mol Sci. 2021;22(2):757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124(1):1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murray IA, Perdew GH. How Ah receptor ligand specificity became important in understanding its physiological function. Int J Mol Sci. 2020;21(24):9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalmes M, Hennen J, Clemens J, Blomeke B. Impact of aryl hydrocarbon receptor (AhR) knockdown on cell cycle progression in human HaCaT keratinocytes. Biol Chem. 2011;392(7):643‐651. [DOI] [PubMed] [Google Scholar]
- 7. Merches K, Schiavi A, Weighardt H, et al. AHR signaling dampens inflammatory signature in neonatal skin gammadelta T cells. Int J Mol Sci. 2020;21(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van den Bogaard EH , Podolsky MA, Smits JP, et al. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J Invest Dermatol. 2015;135(5):1320‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsuji G, Hashimoto‐Hachiya A, Kiyomatsu‐Oda M, et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017;8(7):e2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu X, Zhang X, Zhang J, et al. Activation of aryl hydrocarbon receptor in Langerhans cells by a microbial metabolite of tryptophan negatively regulates skin inflammation. J Dermatol Sci. 2020;100(3):192‐200. [DOI] [PubMed] [Google Scholar]
- 11. Vlachos C, Schulte BM, Magiatis P, Adema GJ, Gaitanis G. Malassezia‐derived indoles activate the aryl hydrocarbon receptor and inhibit Toll‐like receptor‐induced maturation in monocyte‐derived dendritic cells. Br J Dermatol. 2012;167(3):496‐505. [DOI] [PubMed] [Google Scholar]
- 12. Carvajal‐Gonzalez JM, Roman AC, Cerezo‐Guisado MI, Rico‐Leo EM, Martin‐Partido G, Fernandez‐Salguero PM. Loss of dioxin‐receptor expression accelerates wound healing in vivo by a mechanism involving TGFbeta. J Cell Sci. 2009;122(Pt 11):1823‐1833. [DOI] [PubMed] [Google Scholar]
- 13. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716‐724. [DOI] [PubMed] [Google Scholar]
- 14. Jin UH, Lee SO, Sridharan G, et al. Microbiome‐derived tryptophan metabolites and their aryl hydrocarbon receptor‐dependent agonist and antagonist activities. Mol Pharmacol. 2014;85(5):777‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong F, Hao F, Murray IA, et al. Intestinal microbiota‐derived tryptophan metabolites are predictive of Ah receptor activity. Gut. Microbes. 2020;12(1):1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to ah receptor activation. Drug Metab Dispos. 2015;43(10):1522‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang W, Yu T, Huang X, et al. Intestinal microbiota‐derived short‐chain fatty acids regulation of immune cell IL‐22 production and gut immunity. Nat Commun. 2020;11(1):4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosser EC, Piper CJM, Matei DE, et al. Microbiota‐derived metabolites suppress arthritis by amplifying aryl‐hydrocarbon receptor activation in regulatory B cells. Cell Metab. 2020;31(4):837‐851 e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moura‐Alves P, Puyskens A, Stinn A, et al. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science. 2019;366(6472). [DOI] [PubMed] [Google Scholar]
- 20. Schiering C, Wincent E, Metidji A, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542(7640):242‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Metidji A, Omenetti S, Crotta S, et al. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity. 2019;50(6):1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdelsalam NA, Ramadan AT, ElRakaiby MT, Aziz RK. Toxicomicrobiomics the human microbiome vs. pharmaceutical, dietary, and environmental xenobiotics. Front Pharmacol. 2020;11:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong F, Perdew GH. The aryl hydrocarbon receptor as a mediator of host‐microbiota interplay. Gut. Microbes. 2020;12(1):1859812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sherwin E, Bordenstein SR, Quinn JL, Dinan TG, Cryan JF. Microbiota and the social brain. Science. 2019;366:6465. [DOI] [PubMed] [Google Scholar]
- 25. Yip W, Hughes MR, Li Y, et al. Butyrate shapes immune cell fate and function in allergic asthma. Front Immunol. 2021;12:628453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut‐skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9(2):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang DD, Nguyen LH, Li Y, et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med. 2021;27(2):333‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fouladi F, Bailey MJ, Patterson WB, et al. Air pollution exposure is associated with the gut microbiome as revealed by shotgun metagenomic sequencing. Environ Int. 2020;138:105604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leung MHY, Tong X, Bastien P, et al. Changes of the human skin microbiota upon chronic exposure to polycyclic aromatic hydrocarbon pollutants. Microbiome. 2020;8(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bosman ES, Albert AY, Lui H, Dutz JP, Vallance BA. Skin exposure to narrow band ultraviolet (UVB) light modulates the human intestinal microbiome. Front Microbiol. 2019;10:2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conteville LC, Vicente ACP. Skin exposure to sunlight: a factor modulating the human gut microbiome composition. Gut. Microbes. 2020;11(5):1135‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rademacher F, Simanski M, Hesse B, et al. Staphylococcus epidermidis activates aryl hydrocarbon receptor signaling in human keratinocytes: implications for cutaneous defense. J Innate Immun. 2019;11(2):125‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guenin‐Mace L, Morel JD, Doisne JM, et al. Dysregulation of tryptophan catabolism at the host‐skin microbiota interface in hidradenitis suppurativa. JCI Insight. 2020;5(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu J, Luo Y, Zhu Z, et al. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J Allergy Clin Immunol. 2019;143(6):2108‐2119 e2112. [DOI] [PubMed] [Google Scholar]
- 35. Magiatis P, Pappas P, Gaitanis G, et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J Invest Dermatol. 2013;133(8):2023‐2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uberoi A, Bartow‐McKenney C, Zheng Q, et al. Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. bioRxiv. 2021;2020:2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Findley K, Oh J, Yang J, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498(7454):367‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saunte DML, Gaitanis G, Hay RJ. Malassezia‐associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol. 2020;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edslev SM, Andersen PS, Agner T, et al. Identification of cutaneous fungi and mites in adult atopic dermatitis: analysis by targeted 18S rRNA amplicon sequencing. BMC Microbiol. 2021;21(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Corzo‐Leon DE, MacCallum DM, Munro CA. Host responses in an ex vivo human skin model challenged with malassezia sympodialis. Front Cell Infect Microbiol. 2020;10:561382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136(11):2260‐2269. [DOI] [PubMed] [Google Scholar]
- 42. van den Bogaard EH , Bergboer JG, Vonk‐Bergers M, et al. Coal tar induces AHR‐dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123(2):917‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Furue M, Hashimoto‐Hachiya A, Tsuji G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int J Mol Sci. 2019;20(21):5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137(10):2110‐2119. [DOI] [PubMed] [Google Scholar]
- 45. Smits JPH, Ederveen THA, Rikken G, et al. Targeting the cutaneous microbiota in atopic dermatitis by coal tar via AHR‐Dependent induction of antimicrobial peptides. J Invest Dermatol. 2020;140(2):415‐424. [DOI] [PubMed] [Google Scholar]
- 46. Fyhrquist N, Muirhead G, Prast‐Nielsen S, et al. Microbe‐host interplay in atopic dermatitis and psoriasis. Nat Commun. 2019;10(1):4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chng KR, Tay AS, Li C, et al. Whole metagenome profiling reveals skin microbiome‐dependent susceptibility to atopic dermatitis flare. Nat Microbiol. 2016;1(9):16106. [DOI] [PubMed] [Google Scholar]
- 48. Kyoreva M, Li Y, Hoosenally M, et al. CYP1A1 enzymatic activity influences skin inflammation via regulation of the AHR pathway. J Invest Dermatol. 2020;141(6):1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hawerkamp HC, Kislat A, Gerber PA, et al. Vemurafenib acts as an aryl hydrocarbon receptor antagonist: Implications for inflammatory cutaneous adverse events. Allergy. 2019;74(12):2437‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Flaveny CA, Perdew GH. Transgenic humanized AHR mouse reveals differences between human and mouse AHR ligand selectivity. Mol Cell Pharmacol. 2009;1(3):119‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith KJ, Murray IA, Boyer JA, Perdew GH. Allelic variants of the aryl hydrocarbon receptor differentially influence UVB‐mediated skin inflammatory responses in SKH1 mice. Toxicology. 2018;394:27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Emmert H, Rademacher F, Glaser R, Harder J. Skin microbiota analysis in human 3D skin models‐"Free your mice". Exp Dermatol. 2020;29(11):1133‐1139. [DOI] [PubMed] [Google Scholar]
- 53. Gaitanis G, Magiatis P, Stathopoulou K, et al. AhR ligands, malassezin, and indolo[3,2‐b]carbazole are selectively produced by Malassezia furfur strains isolated from seborrheic dermatitis. J Invest Dermatol. 2008;128(7):1620‐1625. [DOI] [PubMed] [Google Scholar]
- 54. Sutter CH, Bodreddigari S, Campion C, Wible RS, Sutter TR. 2,3,7,8‐Tetrachlorodibenzo‐p‐dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol Sci. 2011;124(1):128‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takagi S, Tojo H, Tomita S, et al. Alteration of the 4‐sphingenine scaffolds of ceramides in keratinocyte‐specific Arnt‐deficient mice affects skin barrier function. J Clin Invest. 2003;112(9):1372‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hu T, Wang D, Yu Q, et al. Aryl hydrocarbon receptor negatively regulates lipid synthesis and involves in cell differentiation of SZ95 sebocytes in vitro. Chem Biol Interact. 2016;258:52‐58. [DOI] [PubMed] [Google Scholar]
- 57. Muku GE, Blazanin N, Dong F, et al. Selective Ah receptor ligands mediate enhanced SREBP1 proteolysis to restrict lipogenesis in sebocytes. Toxicol Sci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143‐155. [DOI] [PubMed] [Google Scholar]
- 59. Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132(3 Pt 2):751‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kezic S, O'Regan GM, Yau N, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011;66(7):934‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feuillie C, Vitry P, McAleer MA, et al. Adhesion of staphylococcus aureus to corneocytes from atopic dermatitis patients is controlled by natural moisturizing factor levels. MBio. 2018;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124(3 Suppl 2):R7‐R12. [DOI] [PubMed] [Google Scholar]
- 63. Zeeuwen PL, Ederveen TH, van der Krieken DA , et al. Gram‐positive anaerobe cocci are underrepresented in the microbiome of filaggrin‐deficient human skin. J Allergy Clin Immunol. 2017;139(4):1368‐1371. [DOI] [PubMed] [Google Scholar]
- 64. Haarmann‐Stemmann T, Sutter TR, Krutmann J, Esser C. The mode of action of tapinarof may not solely depend on the activation of cutaneous AHR signaling but also on its antimicrobial activity. J Am Acad Dermatol. 2021. [DOI] [PubMed] [Google Scholar]
- 65. Hashimoto‐Hachiya A, Tsuji G, Murai M, Yan X, Furue M. Upregulation of FLG, LOR, and IVL expression by rhodiola crenulata root extract via aryl hydrocarbon receptor: differential involvement of OVOL1. Int J Mol Sci. 2018;19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Niehues H, Tsoi LC, van der Krieken DA , et al. Psoriasis‐Associated Late Cornified Envelope (LCE) proteins have antibacterial activity. J Invest Dermatol. 2017;137(11):2380‐2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Latendorf T, Gerstel U, Wu Z, et al. Cationic intrinsically disordered antimicrobial peptides (CIDAMPs) represent a new paradigm of innate defense with a potential for novel anti‐infectives. Sci Rep. 2019;9(1):3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hidaka T, Ogawa E, Kobayashi EH, et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol. 2017;18(1):64‐73. [DOI] [PubMed] [Google Scholar]


