ABSTRACT
Background and Purpose
Right to left shunt (RLS), from patent foramen ovale (PFO) or elsewhere, is a recognized risk factor for stroke. Current standard of care for RLS diagnosis includes transthoracic echocardiography (TTE) which is insensitive, transesophageal echocardiography (TEE) which is invasive, and transcranial Doppler (TCD) which has excellent sensitivity and specificity for RLS but is heavily operator dependent and expertise is scarce. The purpose of this study was to evaluate the RLS detection rate of a novel robotic‐assisted TCD (ra‐TCD) to standard of care diagnostic techniques, including TTE, TEE, and TCD.
Methods
This is a multicenter, prospective, single‐arm, nonsignificant risk device study of ra‐TCD versus TTE for RLS diagnosis in adult patients who present with neurological signs and symptoms that include embolic stroke or transient ischemic attack on the differential diagnosis. Up to 150 subjects will be enrolled at up to seven centers considering the prevalence of PFO, suboptimal transtemporal windows, and potential dropouts. Enrolled patients will undergo ra‐TCD supine and at 45° in a manner otherwise in line with standard of care TCD bubble technique. The enrolled patients will have undergone TTE, and optionally standard TCD and TEE, per usual care.
Results
The primary efficacy endpoint is percent detection of RLS by ra‐TCD compared against TTE. The primary safety endpoint is the incidence of device‐related serious adverse events.
Conclusions
This is the first multicenter, prospective study evaluating the accuracy, feasibility, and safety of novel ra‐TCD for the diagnosis of RLS as compared to standard of care diagnostics.
Keywords: bubble study, patent foramen ovale (PFO), shunt, transcranial Doppler (TCD), transthoracic echocardiogram (TTE)
INTRODUCTION
Acute ischemic stroke is a leading cause of death and disability in North America 1 and the world. 2 Patients who suffer acute ischemic stroke are routinely surveyed for a source of cerebral embolism. One element of this investigation is to screen for a right to left shunt (RLS), the most common type being a patent foramen ovale (PFO) in the cardiac interatrial septum. PFO is estimated to be present in approximately 25% of the normal population, 3 but is overrepresented in the ischemic stroke population, especially those who are relatively young and without other traditional vascular risk factors. 4 Therefore, effective screening for RLS is a sine qua non of a thorough evaluation for embolic stroke.
Transthoracic echocardiography (TTE) with agitated saline contrast has been the standard diagnostic for screening for RLS in patients with acute ischemic stroke but suffers from low sensitivity (45.1%), 5 making it a poor screening examination despite its widespread availability and noninvasive nature. Transesophageal echocardiography (TEE) is the “gold standard” for PFO diagnosis 6 but is invasive, requires sedating medications to perform thereby disallowing effective Valsalva by the patient, and does not directly visualize extracardiac shunting. Transcranial Doppler (TCD) is very sensitive (96.1%) and specific (92.4%) for the diagnosis of RLS as compared to TTE 5 and is noninvasive but is heavily operator dependent and availability is relatively sparse. TCD is, otherwise, an ideal screening diagnostic for RLS if not for these limitations.
Recently, robotic‐assisted TCD (ra‐TCD) devices, with artificial intelligence‐enhanced signal detection algorithms, have been introduced to clinical research and practice to help mitigate barriers to TCD performance. More specifically, ra‐TCD can detect and maintain optimal cerebral blood flow velocity signals for embolic monitoring in a relatively operator‐independent fashion, with potential to expand the availability of a very sensitive and specific diagnostic for RLS diagnosis. However, the diagnostic accuracy of ra‐TCD has never been prospectively tested against the standard of care TTE, nor other RLS diagnostics of TEE or TCD.
METHODS
This study is a multicenter, prospective, single‐arm, nonsignificant risk, consecutively enrolled diagnostic accuracy device study. One hundred and fifty evaluable subjects with a clinical condition characterized by neurological signs and symptoms that, in the opinion of the investigator, include embolic stroke or TIA in the differential diagnosis will be evaluated with ra‐TCD and standard of care TTE to screen for RLS, including PFO. To ensure the study is adequately powered, up to 150 subjects will be enrolled at up to seven centers in the United States. The estimation of this sample size was based on the prevalence of suboptimal transtemporal windows and potential dropouts. The enrollment period will last up to 15 months. Subjects’ participation in the study will last from 1 to 60 days. Subjects will be identified and may enroll from any phase of healthcare (e.g., inpatient or outpatient).
This methodology conforms to the Standard for Reporting Diagnostic Accuracy Studies (STARD) paradigm. This trial is registered with ClinicalTrials.gov as study NCT04604015.
Patient population—Inclusion and exclusion criteria
A subject must meet all the following inclusions criteria to be enrolled in the study:
Subject is 18 years of age or older.
Subject presents with a clinical condition characterized by neurological signs and symptoms that, in the opinion of the investigator, include embolic stroke or TIA in the differential diagnosis.
Scheduled for TTE study with agitated saline contrast (bubble study) within ±30 days of informed consent.
Subject can successfully perform a Valsalva maneuver (VM).
Subject or legally authorized representative can provide informed consent and comply with the protocol.
A subject cannot be enrolled in the study if any of the following exclusion criteria are met:
Subject has undergone an RLS/PFO closure.
Female who is pregnant or lactating at time of admission
Subjects who underwent partial or full craniotomy/craniectomy within the past 6 months.
Subjects who have a physical limitation preventing TCD/headmount placement.
Study intervention: ra‐TCD
All study sites will have received approval from local Investigational Review Board (IRB) or ethics.
Enrolled patients will receive all standard of care diagnostics per routine clinical practice, including a TTE with agitated saline bubble study. Enrolled patients will optionally undergo a standard of care TCD bubble study and/or TEE at the discretion of the care team.
The ra‐TCD bubble study will be performed at any time but preferably before TTE and TEE (if applicable) bubble studies are performed for blinding purposes and to avoid false positives because of circulating perflutren microbubbles if used during echocardiography. If a TTE bubble study is performed before TCD bubble study, the standard TCD operator will ensure they are blinded to the results of the TTE prior to performing TCD and if any bubbles were administered during echocardiography—agitated saline or perflutren—the TCD will be postponed for at least 24 h and a brief monitoring session (5–10 min), including a saline flush of the intravenous (IV) line used for echocardiography contrast, will be performed prior to the TCD bubble test to be sure there is no lingering contrast.
The ra‐TCD (NovaGuide™ Intelligent Ultrasound, NovaSignal Corp, Los Angeles, CA, USA) study will consist of an initial set‐up and signal search. ra‐TCD will ideally be performed just after a standard of care TCD bubble study, if performed, since the patient will be already practicing and performing Valsalva for the standard of care TCD bubble study. After setup, the system will search for cerebral blood flow velocity signals at depths between 40 and 65 mm. Once the middle cerebral artery signal has been acquired unilaterally or bilaterally, subjects will be monitored for up to 20 min during the delivery of the contrast agent at rest and with VM.
The study subject will be trained in performance of the VM prior to conducting the ra‐TCD study and/or standard TCD (if performed) in accordance with the training module developed by the principal investigators. Agitated saline contrast will be prepared in standard format with 1 ml of air, 9 ml of saline (preferably bacteriostatic) and, if possible, a blush of venous blood from the IV. Then, the 10 ml of air‐saline contrast agent will be injected into, preferably, the right antecubital vein while the subject is at rest and the MCA signal(s) recorded for a period of 60 s. Other IV locations are acceptable and will be recorded. The subject will remain at rest for approximately 3–5 min. Another 10 ml of contrast agent will be injected in the same IV and the VM initiated during injection, sustained for 10 s. The MCA signal will be recorded for a period of 60 s; characteristic MCA waveform morphology change and mean velocity decrease of at least 25% will serve as demonstration of adequate Valsalva effort. This procedure will be repeated with the study subject in the supine position with head of bed raised to 45 degrees.
If bilateral signals are found, the microbubble count will be sum across both vessels. If only a unilateral signal is found, the microbubble count will be doubled for the total count. If no spectrographic signals can be obtained and there are no embolic streaks noted after injection, the results are “indeterminate.” Spencer Logarithmic Scale (SLS) 7 and International Consensus Criteria (ICC) 8 gradings will be collected for ra‐TCD and standard of care TCD.
Standard of care procedures
Echocardiographic studies (TTE and TEE) will be analyzed for binary yes/no RLS on an intention to treat basis unless there is report of an inability to comment on RLS presence or absence in which case results will be considered “inconclusive.” If bubble count is mentioned in the echocardiography report, it will be categorized by 1–10, 10‐20, or >20.
With standard TCD, if bilateral signals are found, the microbubble count will be sum across both vessels. If only a unilateral signal is found, the microbubble count will be doubled for the total count. Standard TCD does not have “inconclusive” results as patients can be monitored from extracranial internal carotid or the basilar artery unlike ra‐TCD. RLS grading with SLS and ICC will be recorded when available.
See Figure 1, a flow diagram of the study.
FIGURE 1.
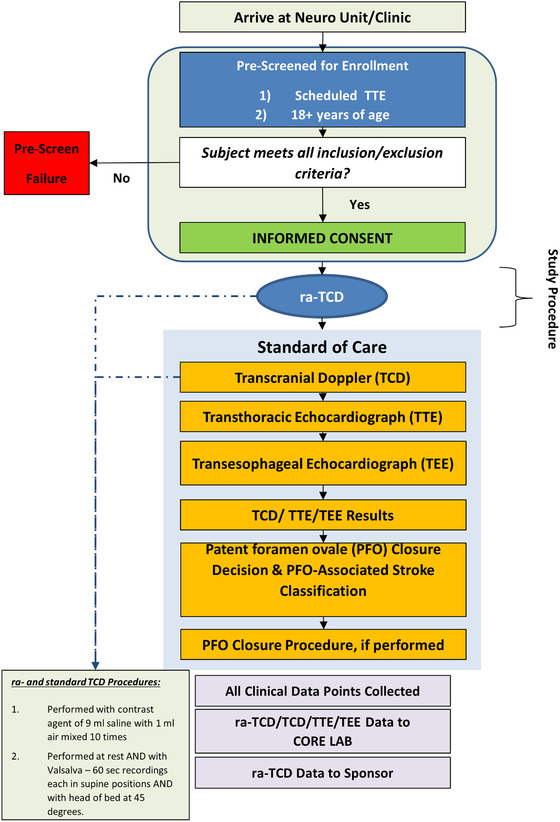
A patient perspective flow diagram of study procedures and analysis
Primary outcome
The primary outcome is the percent shunt detection rate of the ra‐TCD relative to standard of care TTE. The primary safety outcome is the incidence of device‐related serious adverse events. See Figure 2 for a STARD 9 flow diagram of the primary outcome analysis.
FIGURE 2.
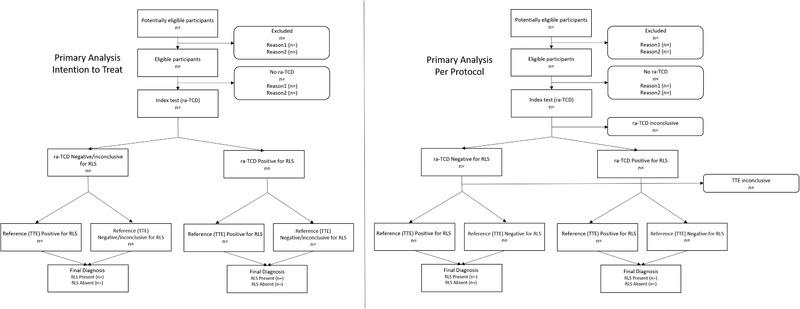
Flow diagrams of the primary analysis, intention to treat, and per protocol. Abbreviations: n, number of subjects; ra‐TCD, robotic‐assisted transcranial Doppler; RLS, right to left shunt; TTE, transthoracic echocardiogram
Secondary outcomes
Secondary outcomes include percent shunt detection rate of the ra‐TCD relative to standard of care TEE and TCD as well as the safety, accuracy, and usability of the ra‐TCD device. See Table 1 for the listing of secondary analyses. See Figure 3 for STARD flow diagram of the primary and main secondary outcome analyses.
TABLE 1.
Secondary outcome analyses
| % detection of false positives, comparison of ra‐TCD with TTE |
| % detection of true negatives (specificity), comparison of ra‐TCD with TTE |
| % detection of false negatives, comparison of ra‐TCD with TTE |
| Positive predictive value (PPV), comparison of ra‐TCD with TTE |
| Negative predictive value (NPV), comparison of ra‐TCD with TTE |
| ra‐TCD diagnostic performance as compared to TEE for diagnostic accuracy parameters (sensitivity, specificity, PPV, and NPV) |
| ra‐TCD diagnostic performance as compared to TCD for diagnostic accuracy parameters (sensitivity, specificity, PPV, and NPV) |
| % detection of intervenable shunts, comparison of ra‐TCD with TTE |
|
| ra‐TCD No Window rate compared to standard TCD (including both unilateral and bilateral absent transtemporal acoustic windows). |
| % success rate of ra‐TCD |
|
| Incidence of device malfunctions |
Abbreviations: ra‐TCD, robotic‐assisted transcranial Doppler; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram.
FIGURE 3.
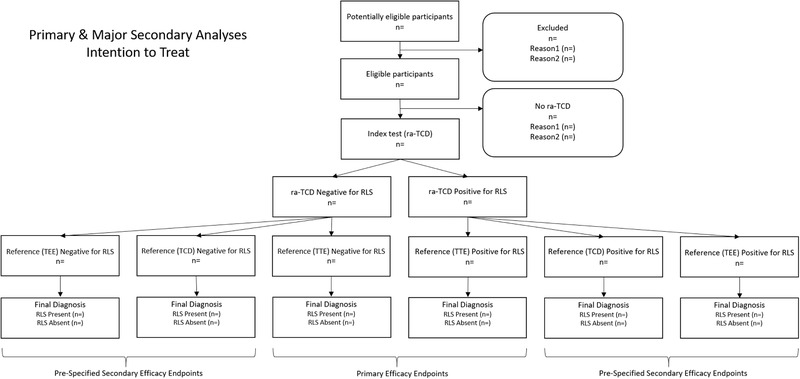
Flow diagram of primary and major secondary intention to treat analyses. Abbreviations: n, number of subjects; ra‐TCD, robotic‐assisted transcranial Doppler; RLS, right to left shunt; TCD, transcranial Doppler; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram
Exploratory endpoints include the Development of an automated algorithm for Spencer Logarithmic Scale and International Consensus Criteria grading of RLS, and a single site (UTHSC) substudy of right heart monitoring with standard technique for quality control and timing of Valsalva during TCD (standard and ra‐TCD).
Statistical analyses
For the primary outcome analysis, the accuracy and sensitivity of ra‐TCD and TTE will be calculated on an intention to treat and per protocol basis.
See Table 1 for secondary outcome analyses.
Exploratory analyses include ra‐TCD automated SLS and ICC grading algorithm diagnostic accuracy and the rate and timing of right heart arrival of bubbles.
All deidentified imaging data will be sent to a core laboratory which will provide independent quantitative and qualitative assessment of all ra‐TCD and standard TCD, TTE, and TEE bubble study data. They will be blinded to the study and local diagnostic report data and provide independent review. The Core Lab interpretations will supersede all local interpretations and will be applied to all study endpoint analyses as applicable.
Data will be analyzed on an intention‐to‐treat basis and thereby any data loss of the ra‐TCD or TTE will be treated as a dropout. As many of the secondary analyses depend on whether or not certain standard of care diagnostics are performed, gaps are expected and will also be analyzed on an intention to treat basis.
Data monitoring body
There is no Data and Safety Monitoring Board (DSMB) because the study is noninvasive and of minimal risk to the patient. Adverse events (AEs) are presumed to be rare in this setting. There is a medical monitor that reviews device‐related AEs and deficiencies quarterly. Procedure‐related AEs related to agitated saline injection before and after VM (headache, allergic reactions, new onset neurological deficit, ischemic stroke, TIA or pulmonary embolism complicating agitated saline contrast injection, etc.) will be closely monitored and prospectively collected but are exceptionally rare. 10
Sample size estimates
The study was powered based on the results of a recent meta‐analysis 5 reporting a pooled TCD sensitivity of 96.1% for RLS detection, while the pooled TTE sensitivity was estimated at 45.1% (absolute difference of 51%). For power calculations, we used a more moderate effect size of 40% increase in the sensitivity of ra‐TCD compared to TTE.
A sample size of 100 subjects achieves 90% power to detect a difference of 40% between two diagnostic tests whose sensitivities are 90% (TCD) and 50% (TTE). This procedure uses a two‐sided McNemar test with a significance level of 0.05. The mean prevalence of PFO in the population of patients with cryptogenic stroke is at least 30%. 11 The proportion of discordant pairs has been set at 0.500.
Given previous reports 10 , 12 , 13 , 14 indicating a prevalence of suboptimal transtemporal windows in 5% of Hispanic, 5% of Caucasian, 9% in African American, and 14% of Asian individuals, we increased our projected sample size by 20% (n = 120). In addition, the final sample size was further increased in order to account for an anticipated dropout rate of at least 20%. Consequently, the final study sample was set at 150 individuals.
Study organization and funding
As the study Sponsor of this clinical study, NovaSignal has the overall responsibility for the conduct of the study, including assurance that the study meets the regulatory requirements of the Food and Drug Administration. The Sponsor will ensure adherence to the regulations as outlined in the Sponsor general duties, selection of investigators, monitoring, maintaining records, and submitting reports.
The steering committee, comprised of a Chair, national Principal Investigator, Sponsor scientific personnel, and all study site Principal Investigators, meet at least monthly.
CONCLUSIONS
This study is the first prospective multicenter, prospective study of the diagnostic accuracy, feasibility, and safety of ra‐TCD as compared to standard of care diagnostics for the diagnosis of RLS.
ACKNOWLEDGMENTS AND DISCLOSURE
Dr. Rubin is a paid consultant of the study sponsor. Dr. Alexandrov is a paid consultant of the study sponsor. Colleen Douville has no disclosures. Brenda Rinsky has no disclosures. Dr. Tsivgoulis is a paid consultant of the study sponsor.
Rubin MN, Alexandrov AV, Douville C, Rinsky B, Tsivgoulis G. Novel robotic TCD ultrasound with bubbles versus standard care to detect right to left shunt: Study methods. J Neuroimaging. 2021;31:858–863. 10.1111/jon.12890
REFERENCES
- 1. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation 2021;143:e254‐743. [DOI] [PubMed] [Google Scholar]
- 2. Lindsay MP, Norrving B, Sacco RL, et al. World Stroke Organization (WSO): global stroke fact sheet 2019. Int J Stroke 2019;14:806‐17. [DOI] [PubMed] [Google Scholar]
- 3. Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17‐20. [DOI] [PubMed] [Google Scholar]
- 4. Elgendy AY, Saver JL, Amin Z, et al. Proposal for updated nomenclature and classification of potential causative mechanism in patent foramen ovale‐associated stroke. JAMA Neurol 2020;77:878‐86. [DOI] [PubMed] [Google Scholar]
- 5. Katsanos AH, Psaltopoulou T, Sergentanis TN, et al. Transcranial Doppler versus transthoracic echocardiography for the detection of patent foramen ovale in patients with cryptogenic cerebral ischemia: a systematic review and diagnostic test accuracy meta‐analysis: TCD vs TTE for PFO. Ann Neurol 2016;79:625‐35. [DOI] [PubMed] [Google Scholar]
- 6. Mojadidi MK, Winoker JS, Roberts SC, et al. Accuracy of conventional transthoracic echocardiography for the diagnosis of intracardiac right‐to‐left shunt: a meta‐analysis of prospective studies. Echocardiography 2014;31:1036‐48. [DOI] [PubMed] [Google Scholar]
- 7. Spencer MP, Moehring MA, Jesurum J, Gray WA, Olsen JV, Reisman M. Power M‐mode transcranial Doppler for diagnosis of patent foramen ovale and assessing transcatheter closure. J Neuroimaging 2004;14:342‐9. [DOI] [PubMed] [Google Scholar]
- 8. Jauss M, Zanette E. Detection of right‐to‐left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc Dis 2000;10:490‐6. [DOI] [PubMed] [Google Scholar]
- 9. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsivgoulis G, Stamboulis E, Sharma VK, et al. Safety of transcranial Doppler “bubble study” for identification of right to left shunts: an international multicentre study. J Neurol Neurosurg Psychiatry 2011;82:1206‐8. [DOI] [PubMed] [Google Scholar]
- 11. Katsanos AH, Giannopoulos S, Frogoudaki A, et al. The diagnostic yield of transesophageal echocardiography in patients with cryptogenic cerebral ischaemia: a meta‐analysis. Eur J Neurol 2016;23:569‐79. [DOI] [PubMed] [Google Scholar]
- 12. Itoh T, Matsumoto M, Handa N, et al. Rate of successful recording of blood flow signals in the middle cerebral artery using transcranial Doppler sonography. Stroke 1993;24:1192‐5. [DOI] [PubMed] [Google Scholar]
- 13. Marinoni M, Ginanneschi A, Forleo P, Amaducci L. Technical limits in transcranial Doppler recording: inadequate acoustic windows. Ultrasound Med Biol 1997;23:1275‐7. [DOI] [PubMed] [Google Scholar]
- 14. Brunser AM, Silva C, Cárcamo D, et al. Transcranial Doppler in a Hispanic‐Mestizo population with neurological diseases: a study of sonographic window and its determinants. Brain Behav 2012;2:231‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]


