Abstract
The etiology of many neurological diseases affecting the central nervous system (CNS) is unknown and still needs more effective and specific therapeutic approaches. Gene therapy has a promising future in treating neurodegenerative disorders by correcting the genetic defects or by therapeutic protein delivery and is now an attraction for neurologists to treat brain disorders, like Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, spinal muscular atrophy, spinocerebellar ataxia, epilepsy, Huntington’s disease, stroke, and spinal cord injury. Gene therapy allows the transgene induction, with a unique expression in cells’ substrate. This article mainly focuses on the delivering modes of genetic materials in the CNS, which includes viral and non-viral vectors and their application in gene therapy. Despite the many clinical trials conducted so far, data have shown disappointing outcomes. The efforts done to improve outcomes, efficacy, and safety in the identification of targets in various neurological disorders are also discussed here. Adapting gene therapy as a new therapeutic approach for treating neurological disorders seems to be promising, with early detection and delivery of therapy before the neuron is lost, helping a lot the development of new therapeutic options to translate to the clinic.
Keywords: Gene therapy, Neurological disorders, Alzheimer’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis, Spinal muscular atrophy
Introduction
Neurological disorders are still a major challenge for physicians because of their multifactorial and multicausal pathogenesis [1, 2]. Treatment of nervous system disorders with classical, ongoing therapies and surgical interventions remains strenuous for several reasons, namely, the complex nature of the central nervous system (CNS), blood–brain barrier (BBB), and the slow regenerative capacity of the nerve tissue. In particular, BBB restricts the entry of many potentially important molecules with high lipophilicity and molecular weight [1–4]. These molecules require intracerebroventricular injections or intracerebral or usage of osmotic mini pumps. Also, neurons that are morphologically and physiologically heterogeneous compared to any other cells in the human body function as convolutions capable of receiving and transmitting signals or information [1, 2, 5].
Gene therapy has appeared as a new therapeutic intervention and an attractive option to deliver genetic material. Gene therapy approaches the treatment of a disorder by introducing a stable and inducible transgene that will correct and replace the defective gene with a controlled expression of the disease environment [6]. Preliminary results were promising that they prompted many investigators to submit protocols for phase I and II trials to many neurological diseases. Recent developments, like neuroimaging, have helped assess precise knowledge about the anatomical-functional relation in assisting clinical evaluation [7, 8]. Indeed, the efficiency of gene therapy for treating CNS diseases must be demonstrated by numerous preclinical and clinical studies [9, 10]. Also worthy of note is that the practice of gene therapy needs several factors to be optimized, like selection of a suitable vector, transgene, and an appropriate delivery mode. The complexity of nervous tissue, the interaction of the host immune system to vector and transgene, makes the practice of gene therapy challenging in neurodegenerative disease. In addition to this, a therapeutic strategy via gene therapy involves challenges such as optimum delivery of the therapeutic agent, which can be possible either by intracerebral delivery or directing growth factors or therapeutic agents stimulating the synthesis of growth factors into the brain parenchyma [11]. Though to be used with caution, growth factors show to be promising in preclinical studies and need to get through in all the phases of clinical trials. Unlike other neuroprotective agents, growth factors work via apposite molecular pathways and involve restoration, protection, and generation of neurons and their functionality. Despite having a shorter half-life, growth factors are capable of activating respective receptors that trigger a cascade of reactions directing the second messengers in the activation of transcription factors, the effect of which can last from days to months post-growth factor inactivation [12]. Additionally, two approaches are noteworthy to be mentioned. One is the amalgam of stem cell and gene therapy that could prove useful in treating neurodegenerative disorders via modification of expression of ectopic protein within the transplanted cells [13]. Another is the improvement of outcomes of human pluripotent stem cells (hPSC) graft via neurotrophic gene therapy, an example of which is the delivery of glial cell line–derived neurotrophic factor (GDNF) that promoted the recovery of hPSC-generated dopaminergic neurons in Parkinson’s disease (PD) [14].
In this article, we mainly canvass the various approaches and vectors used in gene therapy, as also preclinical and clinical studies carried out to treat the various neurological disorders. Besides, the advances stated in gene therapy for the treatment of various neurological conditions, among them PD, Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), spinocerebellar ataxia, epilepsy, Huntington’s disease (HD) and stroke, spinal cord injury (SCI), traumatic brain injuries, and COVID-19-associated neurological conditions, are also highlighted.
Gene Therapy: an Overview
Gene Therapy Approaches
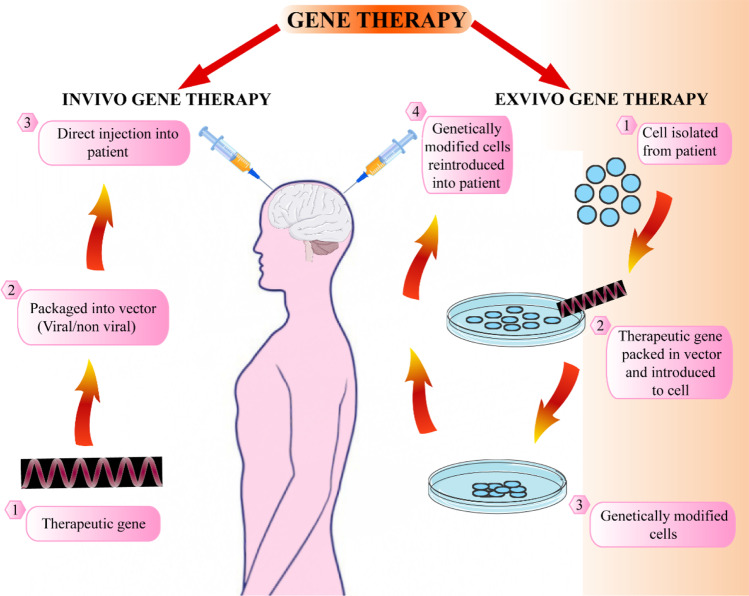
In vivo and ex vivo gene therapy are the two approaches used in gene therapy, by which genes are transferred (Fig. 1). In the in vivo gene therapy, a new gene with the help of a plasmid or viral vectors is directly introduced into the patient, and now, it is further developed utilizing clustered regularly interspaced short palindromic repeats (CRISPR) strategy [15, 16]. The ex vivo gene therapy uses in vitro cell modification, and these cells are transplanted for a stable or transient graft based on the patient to serve the purpose of replacement of faculty cells or providing therapeutic proteins [17–19]. Various in vivo gene therapy complications include the viral vector associated with nonspecific gene expression and targeting insertional mutagenesis, gene silencing, and immune responses against the vector gene silencing and immune responses against the vector [20]. The in vivo gene therapy can also produce strain to CNS cells to work hard making therapeutic molecules. In ex vivo gene therapy, modified cells’ characterization is done before introducing to the patient, and the patient is not directly exposed to the vector [21]. Recent advancements in neural stem cell (NSC) strategies, including the capability to generate autologous induced pluripotent stem cells (iPSC) from the patient’s blood or skin, seem promising in the future for ex vivo gene therapy [22, 23]. The cells can undergo differentiation to produce therapeutically relevant tissues, including oligodendrocytes or astrocytes, besides providing the missing or beneficial protein. In ex vivo gene therapy, fibroblasts and mesenchymal stem cells (MSC) were studied earlier but had many disadvantages because they are not endogenous to the CNS [24–26]. MSC was studied as they show good immunomodulatory activity and generate growth factors and cytokines producing angiogenesis and tissue repair [27, 28], but MSC cannot penetrate the blood–brain barrier (BBB) and cannot survive for long, requiring prolonged administration for long-term effects. The neural progenitor cell (NPC) or NSC can be obtained from many regions of the brain. The self-renewal is limited for NPC and produces neurons and astrocytes [29]. The NSC can differentiate to form oligodendrocytes, astrocytes, or neurons [30]. The human embryonic stem cells is another cell type that can be utilized for ex vivo gene therapy but is associated with ethical problems regarding their derivation [31, 32]. The iPSC can circumvent embryonic stem cells’ ethical issues and are capable of autologous CNS transplantation [33, 34]. Besides the ex vivo gene therapy, non-viral strategies seem promising and can provide protein expression for the long term in non-dividing cells [35, 36]. The recent developments including gene editing strategies such as CRISPR-Cas-9, transcription activator-like effector nucleases (TALEN), and zinc finger nucleases (ZFN) can be employed for the purpose of gene therapy [37]. The strategies depend on genomic site-specific double-stranded breaks that can make possible a precise gene knockin to a sage harbor locus [38, 39]. These gene editing strategies can be utilized and are promising in the future for the therapy of hereditary disorders such as HD as well as the hereditary types of ALS and PD [40].
Fig. 1.
Illustration of gene therapy approaches
Vectors Used in Gene Therapy
The transgene introduction into a vector is a complex procedure, and vectors must possess salient features [41–43], like:
-
i.
The vector must allow the easy manipulation for recombinant technology followed by propagation in suitable hosts.
-
ii.
The vector should possess minimum invasiveness with high cloning capacity. The vector should enable the adaptation of regulatory genes or sequences that ensure the appropriate spatial and temporal regulation of transgene expression and should not have the ability for undesired or uncontrolled alterations of the host genome.
-
iii.
Transgene selected must have exclusive expression only in the target cells.
-
iv.
The vector should be absent from immunogenicity (it should be devoid of genes that bring on immune responses).
-
v.
The vector must allow a prolonged expression of a functional gene that is stable with no alteration in cell progeny.
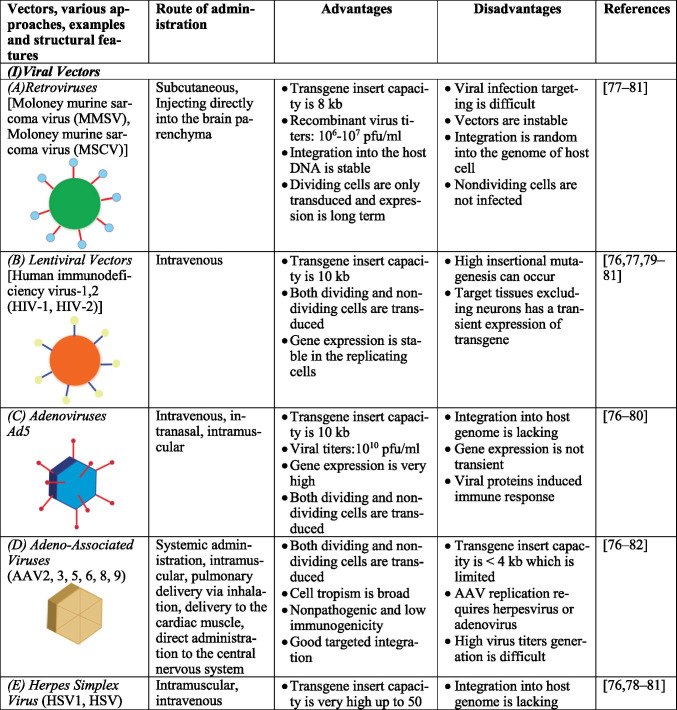
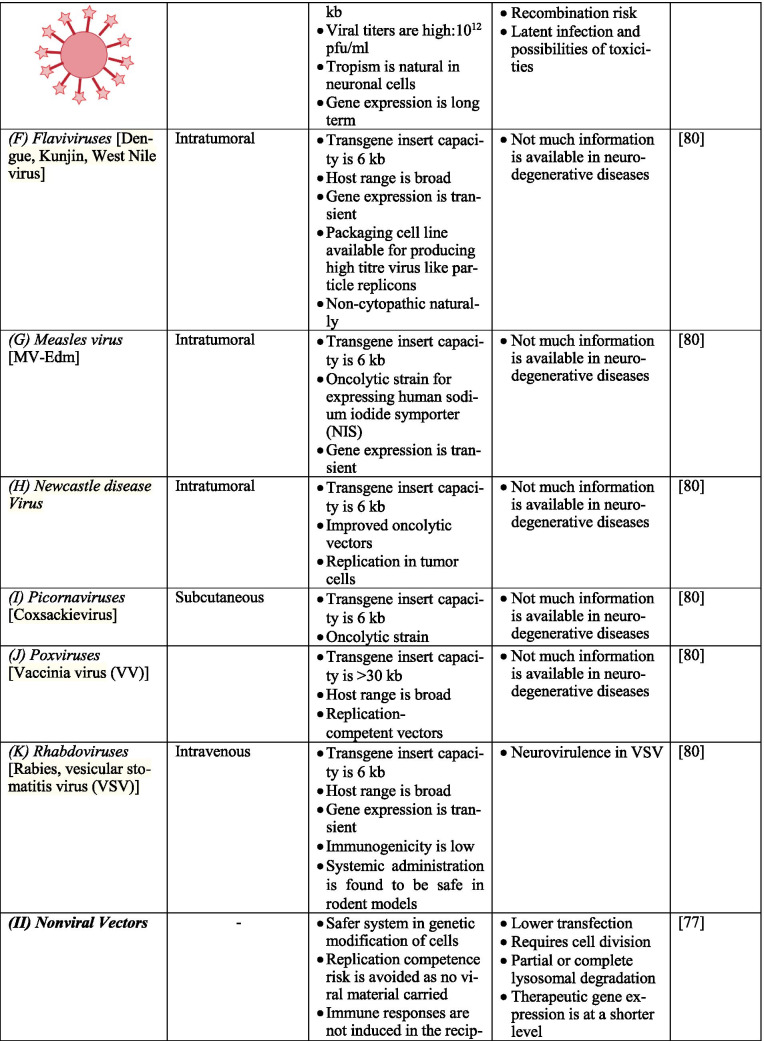
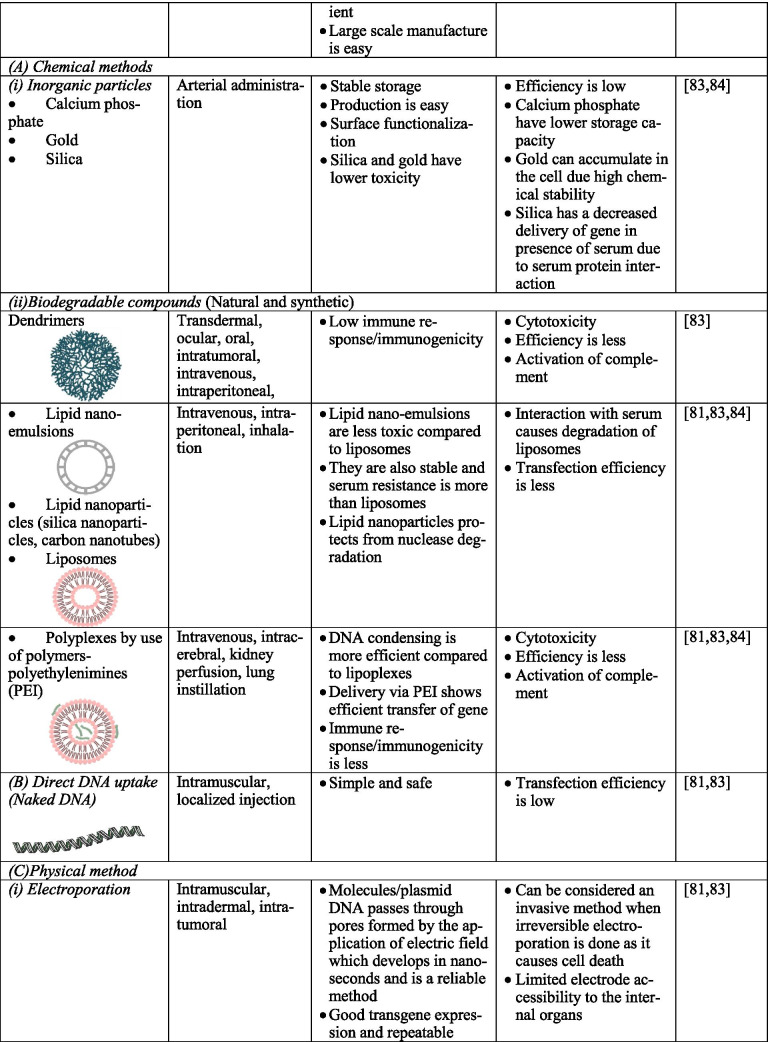
Gene therapy uses different kinds of vectors, like viral and non-viral, which may include synthetic macromolecules, cationic polymers carrying specific ligand for cell surface receptors, and lipid carriers, like liposomes. Viral vectors are a fine strategy to pass and express genetic materials to the host cells. In the CNS, the most commonly used and targeted ones are adeno-associated viruses (AAV), herpes simplex virus-1 (HSV1), retrovirus (RV), and lentiviruses (LV). They can invade cells by triggering infection naturally [44–46]. However, several factors should be considered while using viral vectors, namely, (1) interaction of viral genome with the host genome; (2) antigenicity, putative toxicity, and tumorigenicity of the viral genome; (3) viral tropism for specific genes; and (4) facility of mass production for effective transduction. Interestingly, adenovirus can be regarded as one of the most efficient vectors for CNS due to its ability to divide quiescent cells with high effectiveness and safe usage in approaches such as in vivo and in vitro [47]. For example, adenoviruses deleted with E1 and E3 regions can accommodate large regions and lead to cytotoxicity by viral capsid and inflammation. Also, HSV1 and AAV can infect neuronal cells with high transduction frequency and non-replicating entities; they do not integrate well into the host genome. Additionally, RV infects only dividing cells, and the integration of RV into the host cells’ genome might cause insertional mutagenesis [48, 49]. Table 1 describes various vectors, their structural features, route of administration of vectors, and the merits as well as demerits of each. Figure 2 summarizes the various methods of gene therapy.
Table 1.
Advantages and disadvantages of various vectors.
Fig. 2.
Illustration of various methods of gene therapy
Viral Vectors
Viral vectors are modified viruses that can infect cells and introduce foreign genes. By changing viral vectors, the genes needed for replication are substituted by therapeutic genes. The important viral vectors utilized in gene therapy include RV, adenoviruses (AV), HSV, AAV, and LV. RV are highly effective in transferring genes to the cells that are dividing. The limitation is that they can only infect mitotic cells and have a problem in introducing genes to the post-mitotic neurons. AVs are popular in gene therapy and can be modified by producing viral gene deletions to make space for the foreign gene insertion, thus generating a replication-deficient virus. They can also be utilized to target the non-dividing cells. Their genes do not merge into host chromosomes and are useful in modulating target gene expression.
The neurotropic HSV1 can distribute in the nervous system after infecting the periphery and can be utilized in neurological diseases. They can be utilized to target post-mitotic neurons and are studied for fibroblast growth factor 2 association in neurological diseases. They are utilized to express enzymes important in metabolic disorders, inherited neuropeptides, enzymes synthesizing neurotransmitters, neurotrophic factors (NTFs), and glutamate receptors. The virus’s axonal transport pathway is utilized for transgene expression in the dorsal root ganglion cells or the trigeminal ganglion after subcutaneous injection of the vector [50, 51]. The HSV can be utilized in HD, PD, and AD. The tyrosine hydroxylase gene direct transfer serves as an example. They can also be used in NTF expression for the promotion of peripheral neuron regeneration. AAV can infect post-mitotic neurons and can reach the brain rapidly, thus facilitating targeted gene therapy. Another advantage is that the wild-type virus is not linked to any disease, the capability to infect non-dividing cells, the capacity to introduce a gene into the host’s genome, and transgene expression for a long term. The utilization of AAV vectors is gaining popularity in gene therapy studies [52]. The AAV vectors promote long-term local expression of genes in the CNS.
More than 20 clinical trials have been conducted so far to study the efficacy of AAV vectors in the treatment of neurodegenerative disorders [51, 53, 54]. Table 2 describes clinical trial advances of gene therapy in various neurological disorders. AAV9 is an excellent vector that can be directly introduced into the brain and can produce a global expression in the spinal cord and the brain following a peripheral systemic administration route in animal models [51, 55]. Thus, affecting the entire CNS without being injected into the CNS seems promising for gene therapy. AAVs include serotypes depending on capsid profiles. Multiple AAV serotypes have been identified. They vary in tropism, making each of them suitable for the transduction of specific cells or tissue types with the AAV receptor’s aid. These include AAV1, 2, 3b, 4, 5, 6, 7, 8, and 9 with varying ability to transduce specific cell types. AAV capsid interaction with glycans and proteins in the cell surface as well as the serotypes of varying protein composition is the major factor that determines the efficiency of transduction. AAV1 on direct injection targets local populations of neurons and are administered at high vector doses to exhibit retrograde trafficking activity greater than that of AAV2 and similar to AAV5 and 8. Transduction levels are either higher or similar to that of AAV9 and AAVrh10. AAV1 also exhibits transsynaptic anterograde transport causing post-synaptic neurons to express Cre-dependent transgene [56]. AAV2 also targets local populations of neurons on direct intraparenchymal delivery and capsids of choice for precise targeting. At a high vector dose, AAV2 exhibit retrograde transduction activity lesser than that of AAV1 and AAV5. AAV2 has a lower frequency of transduction in the astrocytes. Modified capsid AAV2-Retro by Tervo et al. show efficient transduction of neurons into the site of injection [56, 57]. AAV4 via intracerebroventricular injection can transduce ependymal cells [58]. Direct intraparenchymal delivery of AAV5 transduces primarily neurons, whereas intraventricular delivery can also transduce multiple regions of the brain including oligodendrocytes and astrocytes [56, 59]. AAV5 exhibits anterograde transduction similar to AAV8 and higher than AAV2 [56]. AAV8 primarily transduces neurons on direct injection and exhibits anterograde trafficking activity higher than AAV2 and similar to that of AAV1 and 5. AAV8 exhibits retrograde transduction on regions such as astrocytes at a low frequency but higher than that of AAV9. Modified capsid AAV MNM008 by Davidsson et al. show efficient transduction of neurons into the site of injection [56, 60]. AAV9 are commonly applied for targeting neurons which exhibit anterograde transduction activity with a transduction level similar to AAV1 and AAVrh10. AAV9 requires high vector doses to exhibit retrograde trafficking activity on astrocytes and at high titers exhibit transsynaptic anterograde transport on oligodendrocytes. AAVrh10 transduces primarily neurons and exhibits both anterograde and retrograde trafficking activity [56]. AAV capsid engineering has been performed to overcome the shortcomings of AAV for better tropism, immunogenicity, and biodistribution. This strategy involves mainly four approaches, rational design, directed evolution, computer-guided design, and a combination of rational and directed evolution [61]. In rational design, only fewer capsid variants are designed, evaluated systematically, and capsid structure refined for desired function which are done based on prior knowledge. Directed evolution involves utilization of random processes including phage display, random insertion of peptides into AAV capsid that are already known, or shuffling of genes of serotypes that are available [62]. In computer-guided design, variant design is conducted based on the knowledge available on DNA sequence and phylogenetic analyses and highly diverse mutants can be generated. But this approach is time-consuming and lacks animal models [61].
Table 2.
Clinical trial advances of gene therapy in various neurological disorders
| Neurological disorders | Gene therapy approaches/route of administration | Clinical trial code | Clinical trials | References |
|---|---|---|---|---|
| Alzheimer’s disease | Approaches by NGF-AAV2 system delivered via basal forebrain | NCT00876863 | Phase 1 and phase 2 | https://clinicaltrials.gov/ct2/show/NCT00876863 |
| AAVrh.10hAPOE2 via intracisternal administration | NCT03634007 | Phase 1 | https://clinicaltrials.gov/ct2/show/NCT03634007 | |
| AAV-hTERT via intravenous and intrathecal administration | NCT04133454 | Both in phase 1 | https://clinicaltrials.gov/ct2/show/NCT04133454 | |
| Parkinson’s disease | LV-AADC via putamen | NCT01856439 | Phase 1 | https://clinicaltrials.gov/ct2/show/NCT01856439 |
| AAV2-GAD via subthalamic nucleus | NCT00643890 | Phase 2 which was later terminated | https://clinicaltrials.gov/ct2/show/NCT00643890 | |
| AAV2-hAADC 2 via striatum | NCT03562494 | Phase 2 | https://clinicaltrials.gov/ct2/show/NCT03562494 | |
| AAV2-hAADC via putamen | Phase 1/2 | |||
| AAV2- NTN via striatum/putamen/substantia nigra/intrastriatal | NCT00985517 | Phase 1/phase 2/phase 2/phase 1 | https://clinicaltrials.gov/ct2/show/NCT00985517 | |
| AAV2- NTN via striatum + putamen | NCT00400634 | Phase 2 | https://clinicaltrials.gov/ct2/show/NCT00400634 | |
| AAV2- GDNF via putamen | NCT01621581 | Phase 1 | https://clinicaltrials.gov/ct2/show/NCT01621581 | |
| Amyotrophic lateral sclerosis | ASO (SOD1) via intrathecal | NCT01041222 | Phase 1 | https://clinicaltrials.gov/ct2/show/NCT01041222 |
| ASO (C9orf72) via intrathecal | NCT03626012 | Phase 1 | https://clinicaltrials.gov/ct2/show/NCT03626012 | |
| Spinal muscular atrophy | Approach by SMN1-AAV9 system via intravenous/ intrathecal | NCT02122952 | Phase 3/phase 1 | https://clinicaltrials.gov/ct2/show/NCT02122952 |
| SMN2 splicing targeted by ASO via intrathecal | NCT02292537 | Phase 3 | https://clinicaltrials.gov/ct2/show/NCT02292537 | |
| Huntington’s disease | AAV5-miHTT via striatum | NCT04120493 | Phase 1/2 | https://clinicaltrials.gov/ct2/show/NCT04120493 |
| ASOs to miHTT pre-messenger RNA via intrathecal | NCT03225833 NCT03225846 | Phase 1 | ||
| Canavan disease | rAAV-Olig001-ASPA via intracerebroventricular route | NCT04833907 | Phase 1/2 | https://clinicaltrials.gov/ct2/show/NCT04833907 |
| Lysosomal storage disorders |
Hematopoietic stem cell gene therapy, lentiviral vector encoding the human ARSA cDNA |
NCT01560182 | Phase 1/2 | https://clinicaltrials.gov/ct2/show/NCT01560182?term=gene+therapy&cond=Lysosomal+storage+disorders&draw=2&rank=1 |
| FLT190- AAV via intravenous infusion | NCT04040049 | Phase 1/2 | https://clinicaltrials.gov/ct2/show/NCT04040049?term=gene+therapy&cond=Lysosomal+storage+disorders&draw=2&rank=2 | |
| Hematopoietic stem cell therapy, CTNS-RD-04) | NCT03897361 | Phase 1/2 | https://clinicaltrials.gov/ct2/show/record/NCT03897361?term=gene+therapy&cond=Lysosomal+storage+disorders&draw=2&rank=5 | |
| X-Linked adrenoleukodystrophy | lentiviral vector TYF-ABCD1 via Intracerebral | NCT03727555 | Phase 1/2 | https://clinicaltrials.gov/ct2/show/NCT03727555 |
| Lenti-D lentiviral vector (ex vivo) | NCT03852498 | Phase 3 | https://clinicaltrials.gov/ct2/show/NCT03852498 | |
| Rett syndrome | Recombinant human insulin growth factor 1 (rhIGF-1) via subcutaneous injections | NCT01777542 | Phase 2 | https://clinicaltrials.gov/ct2/show/NCT01777542 |
AAV1 is capable of transducing skeletal muscles and CNS; AAV2 gets involved in the transduction of a wide range of tissues, including the entire midbrain, skeletal muscles, lungs, liver, and transgene expression occurs at a slower rate. Both AAV4 and 5 can transduce retinal pigmented epithelium (RPE), whereby the AAV5 is to a greater extent than AAV4. In addition to RPE, AAV4 transduces astrocytes and ependyma. Additionally, AAV5 transduces CNS at a higher frequency compared to AAV2 and also photoreceptor cells. AAV6 has a rapid onset of action and has a higher efficiency in transducing skeletal muscles, but VEGF coadministration is a requisite for transgene expression and traversing across the blood vessel barrier. AAV7 has a high transduction profile towards skeletal muscle with a rapid onset of action. Like AAV6, AAV8 has a rapid onset of action in the transduction of skeletal muscle and heart, pancreas, and liver tissue. AAV9, in comparison to AAV2, transduces at higher efficiency tissue of the liver, skeletal muscle, and lungs [63, 64]. Viral vectors move from one area to another in the brain via retrograde or anterograde transport, and the transport depends on the serotype [56, 65]. AAV2 is considered a gold standard in neurosurgical gene therapy because of its phenotype specificity for neurons and the safety profile. It is being studied in several clinical trials [66–72]. AAV2 utilizes anterograde transport, and the downstream targets of neuron projection can produce transgene expression [56, 73]. LV is capable of delivering larger DNA and can also infect post-mitotic cells and exhibit excellent neurotropism. The transgene expression can be studied in a clinical trial for PD [51, 74–76].
Surgical Delivery of Viral Vectors
Convection-enhanced delivery (CED) can directly and efficiently deliver viral vectors in a controlled manner all over the brain [87]. CED employs a pressure gradient to produce infuscate flow in the interstitial fluid space and depends on fluid convection instead of passive diffusion to deliver large-volume, highly concentrated macromolecules. The MRI-guided CED employing intraoperative or interventional MRI can monitor the infusion by administering MRI contrast media and the therapy. Research in primates permitted quantifying CED dynamics and deleting the reflux or leakage [88–91]. Specific areas can be marked for cannula placement [92, 93], and the gadoteridol distribution seen in MRI matches the expression of the transgene [94, 95]. The placement of cannula also depends on the anatomy of individual patients [94, 96]. The commercially obtainable MRI compatible platform for delivery of therapy (MRI interventions, Irvine, USA) includes an aiming device that is mounted on the skull (smart frame), a CED cannula that is resistant to reflux (smart flow), and software that is MRI integrated (clear point) which interacts with the console as well the neurosurgeon performing the procedure. The platform utilizes a two-step design cannula with an inner silica sleeve and a ceramic body enclosed with an outer polymer sleeve. It allows the cannula’s placement to align with the planned route and control the infusion targeted area [11].
Antisense Strategy
The antisense strategy in gene therapy employs agents that modulate the cell’s genetic information processing, especially in diseases caused by genetic abnormalities. Antisense strategy aims to block target protein synthesis in the cell by affecting transcription or translation. Antisense mRNA, which is plasmid derived and introduced with the help of a vector, can produce an arrest of translation. The antisense substances include ribozymes, antigene, and antisense sequences. The oligodeoxynucleotide complementary to DNA or RNA can inhibit targeted protein expression. Ribozymes can catalyze the cleavage of RNA. Antisense therapy can modulate the targeted gene’s function, and antisense drugs can prevent disease-associated protein synthesis. Oligonucleotides are unable to generate proteins but can block the expression of targeted genes. Therefore, antisense oligodeoxynucleotides need to be studied for their potential use in treating neurodegenerative disorders [15, 51].
RNA Trans-splicing
The RNA trans-splicing helps join different pre-mRNA to produce composite mRNA and can help a mutated area of pre-mRNA be substituted with a normal sequence that can code normal proteins. RNA trans-splicing needs to be explored in-depth for its therapeutic utility in neurodegenerative disorders [15, 51].
RNA Interference
The RNA interference (RNAi) is a mechanism that can modulate gene expression and viral replication. The RNA interference, as well as gene silencing, employs the utilization of a double-stranded RNA. According to the sequence, the double-stranded RNAs form small interfering RNAs inside the cell that can recognize and destruct complementary RNAs. RNA interference produces silencing of genes rather than knockout of genes produced by antisense oligonucleotides compared to antisense strategy. Small interfering RNAs are highly efficient and specific and thus can be utilized as antigene agents in gene therapy. RNA interference is being studied in the treatment of ALS. RNA interference can use viral and non-viral vectors for its delivery. RNA interference is promising for its therapeutic potential in treating HD as well as spinocerebellar ataxia in which the associated genes are known, thus can be precisely silenced [51, 97]
Non-viral Vectors
Non-viral vectors utilize cellular mechanisms to introduce DNA then transferring it to the nucleus. An example is the administration of a plasmid DNA utilizing strategies for drug molecules. As in the case of biologicals, the injection has also experimented with gene therapy. The DNA can be introduced into tissues either naked or conjugated with the drug delivery system, including liposomes. In the naked DNA-mediated gene transfer, the naked DNA can be introduced intracerebrally with the help of injection and studied for luciferase expression in mice’s brains. Liposomes can encapsulate the whole of DNA or virus and provide new ways for introducing DNA into human cells. It is a kind of chemical transfection, and the lipid coat helps the cell survival following injection and transfer the DNA by fusing with cells. Liposomes are not that popular because of the sophisticated technique of preparation and efficiency in large fragment encapsulation. Various modifications include the virosome, a liposome with fusogenic viral proteins, and cationic liposomes instead of encapsulation encased by unilateral vesicles with the help of electrostatic force. Cationic liposomes are better than liposomes, and they can bind with a negative charge containing a high-efficiency cell surface, thereby releasing DNA into the cytosol. It is promising in newborns, but in the adult brain, cationic formulations have limited bioavailability. Trojan horse liposome strategy, which conjugates monoclonal antibodies, has been studied in various CNS disorders. They exhibit transcytosis mediated by receptors, penetrating the blood–brain barrier, and endocytosis in the CNS. The technique needs to be studied in the therapy of PD. A synthetic liposome vector with therapeutic DNA can be administered to the systemic circulation and utilized for gene therapy. Ligand-polylysine complexed with DNA can also be utilized for gene therapy. Lipopolyamines are effective transfection agents. The cationic polyamine can complex with the anionic DNA. The polyethylenimine showed excellent transgene expression in the murine brain and showed potential for gene therapy. The molecular conjugate vectors utilize a receptor-mediated mechanism for transferring DNA into tissues. Genes can be targeted to receptors on the cell surface, and DNA can be conjugated to the targeting agent such as polylysine. The DNA released to the cells happens via endocytosis. The artificial chromosomes can be created by combining genomic DNA with alpha satellite DNA synthetic arrays. They contain complete sequence elements for mitotic segregation of chromosomes as well as maintenance. The technique needs to be explored further for its potential role in gene therapy. Non-viral nanoparticles can also be studied for their potential role in gene therapy as they do not have much toxicity and are efficient [98]. Synthetic nano-delivery approach mimicking viruses with non-viral vectors’ safety can be utilized for gene therapy of CNS disorders [51, 99]. The silica nanoparticles have been studied for their potential role in gene therapy. The monodispersed nanoparticle aqueous suspension with the surface-functionalized amino groups for DNA-binding are studied. They can form complex with plasmid DNA and be introduced into the brain, and studies have been done in murine models. Thus, gene nanoparticle complexes can be utilized in neurodegenerative disorders for in vivo gene therapy. Carbon nanotubes can be functionalized and can be made biocompatible to deliver the gene to targeted cells. They can be coupled with dendrimers and can be utilized in gene therapy but need to be studied and standardized. Dendrimers can produce effective neuronal transfection and have low toxicity if the external amino groups undergo surface functionalization. Studies need to be conducted to evaluate BBB permeation’s efficiency and the delivery of genes to glial cells and neurons. This technique also needs further studies to be developed into a gene therapy strategy [51]. The most often used synthetic vectors in gene therapy are cationic polymers and cationic lipids, which permit the electrostatic interaction with DNA [100]. Cationic polymers are like peptides or amino acids positively charged, which can link to ligands ultimately acting at the cell and nuclear level. Also, while cationic lipids are amphiphilic molecules, like cholesterol, that can be infected by in vivo or in vitro methods, the cationic polymer’s efficiency largely depends on the cationic charge and linked stability and saturation [100]. In this way, non-viral vectors, besides being less pathogenic, have the advantage over viral vectors to be of low cost and used in handling techniques [101, 102]. However, to boost transfection effectiveness, non-viral vectors have to overcome intracellular and extracellular barriers [103, 104]. Genetic materials to tissues can be delivered by using physical methods and chemical barriers by microinjection and direct injection [102, 105]. To improve the DNA stability in circulation and release nucleic acids intracellularly, several strategies have been implemented, including the use of acetyl bonds, disulfide bridges, polyethylene alcohol (PEG), and bio-responsive polymers [106–110].
Promoters in Gene Therapy
Gene expression can target certain cells or tissue by the promoter region, active for the long term. Promoter binding varies in bacteria and eukaryotes. Considering eukaryotes, promoter binding is complex to the sense that in order to bind to promoters, RNA polymerase II requires at least 7 transcription factors. The eukaryotic promoters are way complex as well as diverse than the bacterial/prokaryotic promoters. To list out a few eukaryotic promoters in research are CAG (hybrid mammalian promoter), CMV (human cytomegalovirus derived mammalian promoter), EF1α (human elongation factor 1α derived mammalian promoter), PGK (phosphoglycerate kinase gene derived from mammalian promoter), UAS (Gal4 binding sites in drosophila promoter), TRE (Tetracycline response element promoter), and human U6 nuclear promoter (for small RNA expression). Among these, gene expression in TRE is inducible, UAS is specific, and other promoters are constitutive. Bacterial promoters include araBad, lac, trp, Ptac, Sp6, and T7. araBad is an arabinose metabolic operon promoter which is inducible by arabinose. Expression of lac operon–derived promoters is induced by lactose or IPTG, but in absence of lacIq, lacI (lac repressors) are constitutive. trp are E. coli tryptophan-derived promoters which in the presence of tryptophan represses trp gene expression. Ptac are promoters that are hybrid of both trp and lac and are similar in gene expression to that of lac. Sp6 promoters are derived from Sp6 bacteriophage which in the presence of Sp6 RNA polymerase has a constitutive gene expression. T7 promoters are derived from T7 bacteriophage which has a constitutive expression of the gene in presence of T7 RNA polymerase. In short different promoters include constitutive (upregulated promoters that are active throughout various circumstances), repressible (promoters that used to downregulate expression of the gene that can affect the viability of a cell), and inducible promoters (regulated promoters that are active only at specific circumstances or stimuli) [111–115]. Repressible promoters can be either regulated by positive repressible or negative repressible promoters. Positive repressible promoters turn off the transcription by deactivating the activator protein by forming a repressor-activator protein complex and one such example includes Tet-Off. Repressor and co-repressor protein binding promotes the repressor protein to bind to operator and blocking transcription. Such promoters are called negative repressible promoters, and this includes ADH1 which are generally used in yeasts [116, 117]. Inducible promoters can either be positively or negatively regulated. When a positive inducer binds to the activator protein, this in turn binds to the promoter leading to transcription initiation, whereas the negative inducer binds to the repressor protein which in turn detaches from the DNA leading to transcription initiation. Inducible promoters can be mainly of three types, namely, chemical-, light-, and temperature-inducible promoters. Chemically inducible promoters are the most common among inducible promoters. Positive inducible include tetracycline ON (Tet-On) system (used in both prokaryotic and eukaryotic system), LexA promoter (steroid-regulated promoter), and AlcA promoter (alcohol-regulated promoter). Negative inducible include pLac and pBad [118, 119]. Light-inducible promoters activate the expression of genes by using light such as red flame plasmid pDawn which can activate YFI gene responsible for phosphorylation of FixJ. FixJ in turn binds to FixK2 promoter which induces repressor cl transcription. Temperature-inducible promoters express either at a rise in or a fall of temperature and not at regular temperatures. These include heat shock–inducible Hsp70 or Hsp90-derived promoters [120–124]. One can use the promoters for neurofilaments, neuron-specific enzyme D-hydroxylase, enolase, and tyrosine hydroxylase, i.e., glial fibrillary protein astrocytes and myelin basic protein for oligodendrocytes [125, 126]. Sometimes, when the protein quantity is essential, an externally regulated “inducible” promoter is advised. The Tet-off system (tetracycline-controlled transcriptional activation systems) controls gene expression in neurons or astrocytes. Neuron-specific enolase (NSE) promoter driven by the vector herpesvirus was studied for a transgene expression regulation with a conclusion that NSE in herpesvirus vectors is not an optimal promoter that could deliver gene to the CNS neurons. Another approach was NSE incorporated in adenoviral vector in regions of cerebellum, hippocampus, and striatum showed long-term neuronal cell-specific transduction. In spite of being neuron specific, NSE could be expressed in glial cells also. Tetracycline (Tet) regulatable transgene expression (TRE) coupled with adenoviral vector controlled by synapsin I promoter is capable of transfecting rat hippocampus making it significant in glial and neuronal cell function investigations. Synapsin (SYN) I promoter also has a long-term transgene expression from adenoviral vector in regions like thalamus and striatum. Using human cytomegalovirus (CMV) enhancer and platelet-derived growth factor B-chain (PDGF-β), a neuron-specific promoter, neuronal transgene expression can be improved and this can be useful in the study of gene therapy in case of neurological disorders. Cytomegalovirus enhancer and SYN promoter with LV as vector showed persistent neuronal expression. Phosphate-activated glutaminase (PAG) as well as vesicular glutamate transporter-1 (VGLUT1) promoters incorporated into herpesvirus can express glutamatergic neurons, whereas glutamic acid decarboxylase-67 (GAD67) promoter driven by herpesvirus supports expression of GABAergic neuron. Nigrostriatal neuron-specific expression by GDNF or BDNF from herpes simplex virus vectors are useful for investigating gene therapy of Parkinson’s diseases [122].
Promoters in AD
Human PAD gene are promoter of A4 amyloid protein and has close resemblance with that of housekeeping genes possessing 72% GC-rich content in the DNA region. PAD gene regulation can be achieved based on four mechanisms, the GC-rich element involved potential protein binding, CpG region methylation, AP-1/Fos binding site associated with oncogene, and the stress-related heat shock control element [127]. A study by Ohyagi et al. demonstrated the activation of p53 promoter by specific binding of Aβ42 causing possibilities of p53-dependent neuronal apoptosis, synaptic degeneration, mitochondrial dysfunction involved in AD [128]. An Italian case–control study by Bizzarro et al. on APOE promoter interaction in AD confirmed genetic risk factors specifically for ACG3, ATT4, and ATG4 haplotypes, and single-nucleotide polymorphisms (SNP) in APOE promoter gene can be independent of ɛ4 risk factors [129]. Another study reports a weak association of APOC1 promoter polymorphism in AD [130]. Another association is the polymorphism in PIN1 promoter at − 842 (G → C) and − 667 (C → T) regions to have an increased risk of AD [131]. Myeloperoxidase (MPO) gene promoter polymorphism in Chinese Han population has also been reported to have a contribution in AD risk via MPO regulation [132].
Promoters in PD
Based on hypothesis and unbiased (derived from a microarray study) approach, Wettergren et al. made efforts for selection and evaluation of promoter candidates relevant for PD that might prove useful for the disease treatment using gene therapy approaches. Prodynorphin (pDyn), dopamine receptor 1a (Drd1a), and dopamine receptor 2 (Drd2) were selected based on hypothesis approach. From a microarray study angiotensin I converting enzyme (ACE), DnaJ (Hsp40) homolog, microtubule-associated protein 1A (MAP1A), N-Acetylgalactosamine-6-sulfatase (GALNS), and ring finger protein 25 (RNF25) were selected based on unbiased approach. All candidates selected based on both approaches showed more than 90% neuronal specificity and were able to express transgene in rat striatum but the ones selected from microarray study showed highest efficacy [133]. Another study conducted on Prkd1 gene promoter characterization MN9D dopaminergic neuronal cells showed PKD1 to have a neuroprotective role in dopaminergic neurons during oxidative stress at early stages and can probably contribute to PD drug development [134]. Neuron-specific Tα1 α-tubulin (Tα1) promoter induced neuronal-specific expression of Gli1 showed neuroprotective activity. Suwelack et al. concludes that neuronal-specific expression of transcription factors can be specific, more effective, and can apply for targeted neurological gene therapy with minimum side effects [135].
Promoters in ALS
NAD+ in astrocytes activates nuclear factor, erythroid-derived 2, like 2 (Nfe212 or Nrf2) as well as upregulates sulfiredoxin 1 (SRXN1) and heme oxygenase 1 (HO-1). SIRT6 overexpression can also cause Nrf2 activation. Based on these facts, Harlan et al. designed a primer by incorporating Nrf2 binding site onto both Srxn1 and Hmox1 promoters. Enhancing the availability of NAD+ plays a crucial role in modulating various cytoprotective mechanisms and thereby increasing the antioxidant defenses within the astrocytic region which are of importance in motor neuron interaction in ALS. Further studies are required to estimate the therapeutic potential of NAD+ in ALS [136].
Route of Administration
Two routes are preferred to what concerns administration routes, either direct injection or an indirect approach that determines gene therapy’s safety and efficiency. Indirect injection, a restricted and precise gene region, is targeted, which requires stereotaxic guidance, whereas the indirect route can be used for neuron population with a retrograde axonal-transport system [137, 138]. This indirect approach facilitates a selective and targeted transfection of motor neurons by intramuscular injection of the vector carrying a gene of interest. This review gives an account of gene delivery via various routes by utilizing AAV vector.
Systemic Delivery
AAV as a vector can be transduced via systemic circulation targeting the liver and most trials have demonstrated its ability in correcting defective gene mostly inherited monogenic diseases [20]. Localized administration via eye is not influenced by NAb levels, but in parenteral administration, the NAb levels need to be monitored in patients and the re-administration possibility is hard [139]. There are concerns from animal models that rarely rAAV vectors integrate into the genome producing genotoxicity [140, 141]. In hepatocellular carcinoma samples, the AAV sequences were present adjacent to cancer driver genes, even though rarely [142]. These concerns need to be addressed and the possibility of genotoxicity should be monitored in the development of AAV vectors.
Intramuscular Administration
The direct intramuscular administration via injection is another delivery strategy. In Europe, Glybera is an approved AAV gene therapy strategy which is an AAV1 that codes lipoprotein lipase deficiency gene [143, 144]. Various AAV variants can effectively target transduction of skeletal muscles [145]; then, after transduction, the muscle cells act as a site for protein production which produces its effect either locally or systemically. The cell turnover in case of muscle cells is low, so that the transduced AAV will remain in these cells as an episome for many years and is evident in research with primates [145]. Therefore, a single-dose intramuscular administration does not require future re-administration unless the transduced product undergoes immune clearance or is severely damaged. The therapeutic strategy is being utilized in AGTC as well as Adverum for muscular dystrophy as well as α1-antitrypsin deficiency [20].
Central Nervous System Administration
In Parkinson’s disease and in case of inherited Canavan disease, Batten disease, as well as mucopolysaccharidosis (MPS) IIA, IIB, IIIa (Sanfilippo syndrome type A) and IIIb (Sanfilippo syndrome type B), direct administration to the CNS is employed. Various phase I/II trials are being currently carried out utilizing AAV variants such as AAV2, AAV9, and AAVrh10 [54, 145, 146]. The strategies for administration include direct intraparenchymal delivery to various brain regions as well as utilizing other routes such as cisternal, intracerebroventricular, and lumbar intrathecal route [146]. The ideal route for administration depends upon the disease condition as well as the targeted areas. In the case of PD, the current knowledge recommends direct injection into the striatum, substantia nigra, or the putamen. In case of diseases affecting major regions of the brain, for example, MPS and Canavan disease, an injection to cerebellum is employed [146, 147]. A direct delivery to the cerebrospinal fluid (CSF) utilizing intrathecal route produces wide distribution in the CNS and can be employed in cases of AD as well as spinal muscular atrophy [146–150]. The AAV variants which can permeate the BBB can be delivered systemically as a substitute for administration into the CSF. The AAV9 can permeate the BBB and transduce to wide areas of the CNS [45, 148, 151] and is being utilized by AveXis (AVXS-101) in the therapy of spinal muscular atrophy.
In AD, the neurofibrillary tau tangles (NFTs) as well as amyloid plaques, which are neurotoxic, have been aimed to be cleared utilizing antibodies which are plaque specific, showed promising result of limitation of disease progress in various animal experiments as well as early clinical studies [152, 153]. But larger research showed the results to be inconclusive. Failures where are also documented which can be because of inadequate exposure of brain areas to the antibody therapy [153, 154]. Alternate approaches can utilize administering AAV which encodes the antibody, ether via direct local administration to the CNS or systemic administration, then reaching the brain via BBB permeation, producing high exposure of the CNS to the therapeutic antibody [155].
Cardiac Administration
The direct local administration of AAV to the cardiac muscle has been studied for heart failure in several clinical studies. The SERCA2A have been tried to be delivered to the heart, but the study by Celladon failed to yield positive results. Another study by UniQure aims to deliver S100A into the heart and is under preclinical studies [156–158]. Celladon utilized intracoronary infusion to administer the SERCA2A AAV1 product, whereas UniQure utilizes retro-infusion as well as left anterior descending coronary occlusion [158, 159]. The strategy can target S100A AAV9 product to the area of interest in the heart and the real benefit will be understood in future studies.
Pulmonary Administration
The AAV as aerosolized form fore inhalational pulmonary administration were studied for cystic fibrosis (CF) but were unsuccessful in producing positive results yet indicated the safety of AAV via the route of administration [160–162]. Airway congestion can affect AAV distribution hampering the transduction (118). The CF transmembrane conductance regulator (CFTR) gene has a size of more than 4 kb; the upper limit of AAV package capacity and the gene is expressed in submucosal glands which is difficult to be targeted effectively [160, 161]. Despite all these facts, the efforts showed AAV as a safe delivery strategy targeting the lungs and can be utilized in other diseases such as influenza as well as other lung infectious diseases [163]. The local delivery of AAV as a gene therapy strategy is just in the beginning stages of exploration. The broad tropism as well as the viral stability in various cells and tissues can be utilized efficiently. At least an AAV variant can be utilized for each targeted tissue type and AAV discovery as well as engineering can generate AAV variants with desirable specialized functions. All these efforts can generate novel therapeutic approaches for newer indications [54].
Gene Therapy in Neurological Conditions
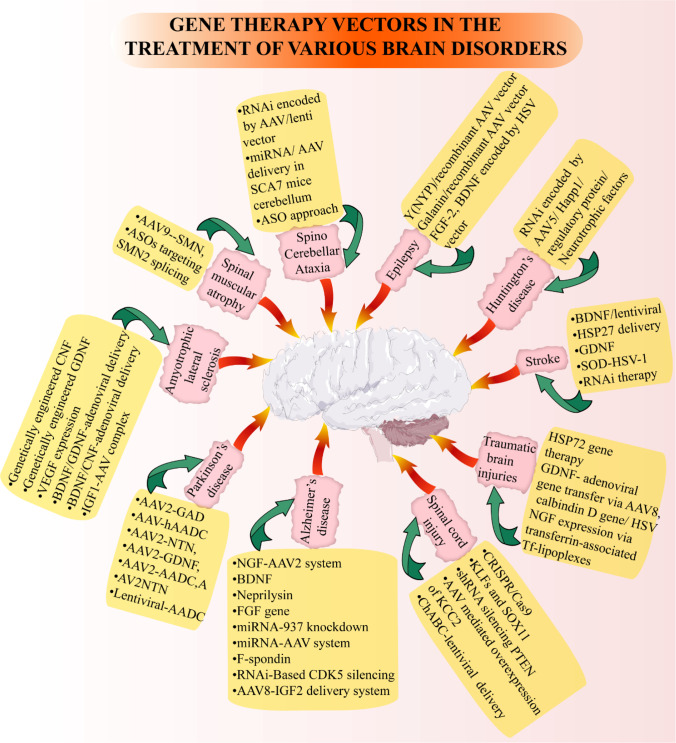
Gene therapy has been identified as a key therapeutic strategy for nervous system disorders. Despite the advances stated so far, there is still a gap between principal theories and therapeutic efficiency. The initial trial identified that gene therapy is a safe method. A well-tolerated vector identified is AAV-9, with a robust neuronal tropism and cross BBB, besides revealing highly effective in various preclinical and clinical trials [164–167]. Another commonly used viral vector is a tricistronic LV vector, prosavin running in a clinical trial to treat PD [75, 168]. Several delivery methods have also been used, like intracerebroventricular injection, intrathecal, direct delivery to the brain, and spinal delivery, mainly depending on the transgene efficiency [169–172]. A better understanding of the neurological diseases’ etiology and the identification and validation of a reliable biomarker will give a clearer idea of disease initiation and progression. Table 3 gives an account of gene therapy approaches including growth factor gene therapy and the possible mechanisms/outcomes in various neurological disorders. The various brain disorders and vectors used in gene therapy are described in the next subsections and summarized in Fig. 3.
Table 3.
Gene therapy approaches in various neurological disorders
| Neurological disorders | Gene therapy approaches | Outcomes/mechanism | References |
|---|---|---|---|
| Alzheimer’s disease | Growth factor gene therapy approaches | ||
| Nerve growth factor (NGF)-AAV2 system | Restoration of cognitive capabilities | [173, 174] | |
| Brain-derived neurotrophic factor (BDNF) | Improvement in cognitive abilities and synaptic plasticity in transgenic mice | [175, 176] | |
| Fibroblast growth factor (FGF) gene | Clearance of hippocampal Aβ and significant improvement in spatial learning | [177] | |
| AAV8-insulin-like growth factor-2 (AAV8-IGF2) delivery system | Reduction in the Aβ levels, forming of dendritic spine is promoted and memory enhanced | [77, 178] | |
| Miscellaneous gene therapy approaches | |||
| LV-PGRN | Progranulin haploinsufficiency overexpression leads to inhibition of spatial memory dysfunction and neuronal loss | [179] | |
| AAV1-AKT | Restore aberrant mTORC1 activity thereby preventing neurodegeneration | [180] | |
| AAV2-PINK1 | Overexpression of PINK1 causes promotion of autophagy by facilitating dysfunctional mitochondria clearance there by ameliorating decline in cognition and Aβ induced synapses | [181] | |
| AAV2-PSD95-6ZF-VP64 | Epigenetic regulation and promotion of autophagy | [82] | |
| AAV2/8-sTREM2 | TREM overexpression leads to an improvement in the migration and proliferation of microglia and Aβ degradation, thereby spatial memory dysfunction is ameliorated | [82] | |
| Neprilysin | Reduction in the Aβ levels and improvement in synaptic density and alleviation of AD pathology in transgenic mouse models | [182] | |
| miRNA-937 knockdown | Antisense miRNA-937 overexpression in MSC which increased Brn-4 expression responsible for neurons development | [21] | |
| miRNA-AAV system | Lowering of Aβ levels in mouse | [183] | |
| F-spondin | Administration into the dentate gyrus of the hippocampus of mice, it showed reduced amyloid plaques and increased learning memory | [77, 184] | |
| RNAi-based therapy | CDK5 silencing by using RNAi probably suppresses neurofibrillary pathology and τ hyperphosphorylation | [185] | |
| Parkinson’s disease | Growth factor gene therapy approaches | ||
| Neurturin (NTN) | Binds to the GFRα 1 and 2 of the GDNF receptor and is a structural and functional homolog of GDNF having similar neuroprotective nature that of GDNF in ameliorating PD pathology | [186, 187] | |
| Glial-derived neurotropic factor (GDNF) | These are neurotrophins that via AAV-mediated gene transfer caused minimal putamen coverage whereas via lentiviral delivery resulted in reduction of cytokines in substantia nigra and striatum and microglia in the striatum of MPTP lesioned and normal monkeys | [17, 188] | |
| AAV2-GAD | Primarily alteration in Unified Parkinson’s Disease Rating Scale (UPDRS) which was later terminated due to financial reasons, and this system works by inhibiting local GABA, thereby correcting the pathological hyperactivity in basal ganglia | [11, 187] | |
| Miscellaneous gene therapy approaches | |||
| AAV1-AKT | mTOR signaling activation whereby restoration of aberrant mTORC1 activity occurs thereby preventing neurodegeneration | [82, 189] | |
| AAV2-hAADC | hAADC delivery to MPTP-lesioned primates caused long-term and significant improvements in behavioral rating scores. Therapeutic goal of therapy strategy was to produce a continuous as well as stable production of dopamine in the motor region of the putamen | [11] | |
| AAV2-HSP70 | Overexpression of HSP70 regulates mitochondrial oxidative stress and functions thereby reducing neurotoxicity | [82] | |
| AAV2-TFEB | Transcription factor EB overexpression enhances neuronal survival and axonal regeneration thereby improving α-synuclein-induced neurodegeneration | [82] | |
| AAV2-XBP-1 | Local delivery of XBP-1 to substantia nigra or striatal region can halt neurotoxin induced neurodegeneration | [82, 190] | |
| AAV5-BiP | BiP overexpression reduces ER stress and unfolded protein responses thereby reducing apoptosis of dopaminergic neurons as well as progression of disease, addition to motor performance enhancement | [82, 191, 192] | |
| AAV6-Lamp2a | Lysosome-associated membrane protein 2a overexpression enhances neuronal survival and axonal regeneration thereby improving α-synuclein-induced neurodegeneration | [82] | |
| RNAi Therapy | α-Synuclein suppression or knock down by RNAi can effectively treat PD. Another approach is β-synuclein encoded gene transfer which will bind to α-synuclein thereby cause reduction in accumulation and aggregation of α-synuclein in synaptic membrane | [193] | |
| Amyotrophic lateral sclerosis | Growth factor gene therapy approaches | ||
| Genetically engineered cells secreting ciliary neurotrophic factor (CNTF) | Beneficial effects of NTFs, not complete cure | [194, 195] | |
| Genetically engineered myoblast cells secreting GDNF | Promotes survival of motoneurons, thereby delaying neurodegeneration of ALS | [196] | |
| BDNF/GDNF-adenoviral delivery | Massive motor neuron death was prevented | [197] | |
| BDNF/CNTF-adenoviral delivery | Axotomized motor neurons survived up to 5 weeks, neurotrophin-3 overexpression could prevent axonal degeneration at motor end plates but effect was little in the quantity of neuronal cell bodies in motor end plates | [198] | |
| IGF1-AAV complex | Causes retrograde transportation from spinal muscles to motor neurons thereby prolonging life and delaying progression of the disease | [199] | |
| Miscellaneous gene therapy approaches | |||
| AAV6-SIL1 | SIL1 delivery to intracerebral region reduces ER stress by restoration of ER homeostasis thereby prolonging survival | [82] | |
| AAV9-snapin | Snapin overexpression aids in reversing autophagy impairment, survival of motor neuron enhancement by the correction of retrograde transport defects | [82] | |
| Vascular endothelial growth factor (VEGF) | VEGF expression via lentiviral vector could delay the onset as well as progression of ALS | [200, 201] | |
| Spinal muscular atrophy | Replacement of SMN1 via AAV9 | Most animal studies implicate a delay in disease progression, but only the partial progression in motor neuron numbers | [202–204] |
|
Spinocerebellar ataxia Epilepsy Huntington’s disease Stroke Spinal cord injury Traumatic brain injuries |
RNAi therapy | SCA1 and SCA7 are targeted through direct brain injection via RNAi. SCA1 targeted by RNAi causes suppression of polyglutamine-induced neurodegeneration. Suppression of Atxn3 in SCA3 rats via lentiviral delivery demonstrated mitigation of degeneration | [205] |
| miRNA delivery | miRNA delivery in SCA3 mice caused improvement in molecular phenotype. The usage of miR-3191–5p in SCA6 animal models demonstrated protection against Purkinje cell degeneration, motor deficits, and ataxia. Direct delivery of miRNA or AAV in SCA7 mice cerebellum showed an improvement in ataxia phenotype | [205] | |
| Antisense oligonucleotide ASO approach | Targeted against ATXN1, ATXN2, and ATXN3 demonstrated a reduction in these protein levels | [205] | |
| Transfer of neurotrophin genes | FGF-2, BDNF encoded by HSV vector containing transgene can cause neural tissue regeneration and reduce the epileptogenesis | [206–208] | |
| Galanin and neuropeptide Y delivery via recombinant AAV vector | Seizure inhibition | [209–211] | |
| Rearrangement of glutamate or GABA receptor composition to modulate receptors response | Gave promising results in epileptic animal models | [212] | |
| Restoration of neuropeptide balance | CG01 along with NPY and Y2 genes when delivered into the brain post-surgery confirmed antiepileptic effects by restoring neuropeptide balance | [213] | |
| RNA interference (RNAi) |
This approach has aided in suppressing pathological level of polyglutaminated huntingtin (mHtt) protein and delivery via AAV5 has demonstrated a widespread distribution of the transgene Exon 1 of huntingtin gene targeted by adenoviral vector expresses a short hairpin RNA which is capable of inhibiting expression of huntingtin in both non-neuronal as well as neuronal cell lines |
[214–216] | |
| Genetically engineered nerve growth factor-producing fibroblasts | Protection against excitotoxic insults | [217] | |
| Encapsulated genetically engineered cellular implants | Encapsulated recombinant human ciliary NTF implants that produce fibroblasts prevents degeneration of striatum and behavioral defects | [218] | |
| Gene transfer of NGF/CNTF factor | Prevention of degeneration | [217, 218] | |
| Ciliary neurotrophic factor (CNTF)-lentiviral delivery | Showed dose dependent neuroprotective effect in rat model | [218] | |
| AAV-GDNF | Incorporation into mouse striatum developed behavioral as well as neuroanatomical protection | [15, 219, 220] | |
| AAV1-caRheb | Restore aberrant mTORC1 activity thereby preventing neurodegeneration | [82] | |
| AAV2-XBP-1 | Local delivery of XBP-1 to substantia nigra or striatal region can halt neurotoxin induced neurodegeneration | [82, 221] | |
| Antisense therapy | Could effectively cause reduction of mHtt protein | [222] | |
| Growth factor gene therapy approaches | |||
| FGF-2 | Proliferation of progenitor cell leading to neurogenesis | [223] | |
| GDNF | GDNF administration could cause reduction in motor function damage, and cerebral infarction were limited which might have occurred due to antiapoptotic and NTF mechanisms | [224] | |
| Miscellaneous gene therapy approaches | |||
| Stem cell gene therapy approach | NTFs such as BDNF transfects recombinant mesenchymal stem cells delivered by lentiviral vectors can promote recovery and regeneration of neurological function | [225] | |
| HSP27 delivery | HSP27 delivery with a suitable viral vector could reduce the lesion size in experimental stroke model post stroke | [226] | |
| SOD-HSV-1 | Antioxidant gene, SOD when administered through striatal region prior or post cerebral ischemia could improve 50% of neuronal survival due to neuroprotective property | [227] | |
| RNAi therapy | Endothelial gene silencing via T cell invasion could hold the production of neurotoxic cytokine as well as secretion of IF-γ thereby reducing neuroinflammation after ischemia as well as the infarct volume | [228] | |
| CRISPR/Cas9 genome editing | Extenuated the adverse effects caused by spinal cord injury | [229] | |
| Enhancement of pro-regenerative factors | Kruppel-like factors (KLFs) and SOX11 promotes axonal regeneration and neurogenesis | [229] | |
| Short-hairpin RNA (shRNA) silencing inhibitory factors | Phosphatase and tensin homolog (PTEN) via mTOR downregulation can reduce damaged neuron regeneration | [229] | |
| Chloride potassium symporter 5 (KCC2) | KCC2 was identified which could maintain a balance in excitatory and inhibitory neurotransmission ratio via modulation of neural circuits and AAV mediated overexpression of KCC2 could improve the functional recovery with less or no adverse effects by influencing synapsin promoter | [229] | |
| Enzymatic degradation of glial scars secretions |
Chondroitinase ABC (ChABC) causes enzymatic degradation of CSPGs that can improve neuronal functionality and regeneration Application of gene therapy that is by incorporating lentiviral vector to ChABC gene resulted in a long-term expression the gene that could improve fine motor recovery post cervical SCI |
[229] | |
| Gene therapy with heat shock protein (HSP72) | High neuroprotective potential, promotion of growth, survival and differentiation of neuronal cells | [230] | |
| GDNF- adenoviral delivery, gene transfer via AAV8, transfer of calbindin D gene via HSV, NGF expression via transferrin-associated cationic liposome/ Tf-lipoplexes | Caused attenuation in traumatic injury in focal cortex and these include | [231] | |
| AAV-based overexpression of S6K1 or AKT | Therapeutic effect by mTOR signaling activation | [231] | |
Fig. 3.
Application of gene therapy vectors in the treatment of various brain disorders. Various diseases of the brain are represented in pink boxes, and yellow boxes indicate the specific vectors used in gene therapy for the treatment of central nervous system disorders
Alzheimer’s Disease (AD)
AD is a very common progressive neurological disease and is currently considered a social burden. AD is characterized by the damage of brain regions and neuronal circuits by the deposition of amyloid plaques, leading to neuronal circuit dysfunction and degeneration, which ultimately leads to memory loss, resulting in dementia and death. AD is known to be age-related, inherited as an autosomal disorder linked to several genetic risk factors [232, 233]. Indeed, small drifts or mutations are present in several genes, such as the amyloid-beta precursor protein (APP) gene or presenilin genes. AD results in cholinergic neuron loss in nucleus basalis magnocellularis and the inhibitors of cholinesterases include the primary therapy but result in little symptomatic relief. In the basal forebrain, the cholinergic neuron function can be increased by nerve growth factor (NGF). The protective action of NGF has been seen in the primates with lesions and aged primates [25, 234, 235].
Growth Factor Gene Therapy in AD
The AAV2 delivery of NGF was examined in a dose-escalating clinical study phase I in mild to moderate AD patients. The study assessed long-term safety and feasibility utilizing a traditional surgical strategy [25, 234–237]. Earlier studies utilized fibroblasts modified genetically and were examined in primate and rodent models possessing cholinergic injury. The NGF producing fibroblasts survived and increased the cholinergic neuron survival [24]. A direct kind of gene therapy employing AAV2, which encodes NGF, was studied [238]. Studies employing a rodent lesion model for AD utilized NGF expressing modified NSL engrafted in the CNS restoring cognitive capabilities and seems promising for the future [173, 174]. NSC’s BDNF basal production can also notably improve cognitive abilities and synaptic plasticity in AD transgenic mouse models [175, 176]. The genetically modified NSC, which shows BDNF overexpression, showed an effect in the AD transgenic mouse model [239]. An insulin-like growth factor (IGF) producing fetal cortical-derived NSC survived for 10 weeks in the AD transgenic mouse model, but the exact therapeutic effects need to be explored [240]. Modified NSL producing neprilysin, an enzyme degrading Aβ led to a betterment in synaptic density and alleviated AD pathology in transgenic mouse models [182]. Many studies regarding MSC transplantation showed promising outcomes on cognitive capabilities in mouse models of AD producing Aβ reduction, microglial function, and neuroinflammation modulation [28, 241, 242]. A study by Kiyota and team on transfer of FGF2 gene in AD mouse model concluded FGF2 delivery via adeno associated virus serotype 2/1 hybrid (AAV2/1-FGF2) could enhance neurogenesis and hippocampal Aβ clearance which puts forward chances of FGF2 gene delivery to be used as an alternative in AD therapy [243].
Miscellaneous Gene Therapy Approaches in AD
In another study, miRNA-937 knockdown through antisense miRNA-937 overexpression in MSC increased Brn-4 expression, which is important for neurons’ development. In the AD transgenic mouse model, these cells were introduced to the hippocampus, resulting in Aβ reduction, raised BDNF, and improved cognition [21]. In AD, the ex vivo gene therapy is promising a lot of studies are yet required to utilize stem cells as delivery technology to alleviate the pathology of AD modifying stem cells to generate TGF-β growth factors microglial activation modulators or improving the function of astrocytes can be studied for its therapeutic potential [244–247]. A major difficulty can be targeting a wide brain region with cells to generate the desired therapeutic action [15].
A study conducted in a mouse model proved that an AAV expressed miRNA capable of inhibiting acyl CoA: cholesterol acyltransferases 1(ACAT1), capable of lower Aβ levels [248, 249]. Another wild mouse study revealed that antisense oligonucleotide therapy could inhibit microtubule-associated protein τ. But there is an emerging need in assessing animal models of AD to assess the progression of damage [250]. An enzyme neprilysin mediates Aβ catabolism, and its amount is decreased during the early stages of AD. Two papers published using the `AAV9 vector about the administration of neprilysin/membrane metalloendopeptidase (MME) by direct injection into cortex or hippocampus by intracardiac administration [250–252]. These studies gave a reduction in the Aβ levels. Various studies showed that the symptoms of AD could also be lessened by leptin. A double transgenic mouse is injected with a LV vector containing leptin, which reveals a low Aβ load and reduces τ phosphorylation with a better synaptic density [253]. Reel pathway expression has been identified as the therapeutic target for AD, linked to the early pathogenesis as it reduces τ phosphorylation. Other studies on F-spondin, a homologous of Reelin, revealed that when administered into the dentate gyrus of the hippocampus of mice, it showed reduced amyloid plaques and increased learning memory. Apoptosis is caused by the expression and knockdown of various genes. The toxic and ill effects of Aβ generate reactive oxygen species and induce apoptosis [184]. Another study implicated an improvement in learning and memory [254] with low levels of Caspase-3 proteins (CASP3) and apoptosis by induction of LV short hairpin (shRNA) against CASP3. In a later study, it was found that oxidative stress–induced apoptosis and neurotoxicity of Aβ could be attenuated by targeting hypoxia-inducible factor 1 α subunit (HIF-1), which possesses neuroprotective activity [255].
Neurotropic support using growth factors is also evaluated for AD. Animal studies implicate that many of the growth factors showed successful results but failed in clinical trials, showing that the procedure needs more refinement and better targeting. In the triple transgenic mouse model study, it was found to preserve learning and memory but no change in the levels of τ and Aβ [77]. A reduction in the Aβ levels is observed during the overexpression of human APP using the AAV8-IGF2 delivery system [178]. The authors illustrate the link between IGF2 and AD expression by showing the reduced amount of IGF2 in the hippocampus of AD patients. According to scientists, the new targets have promiscuous potential but require fully tested clinical trials to estimate the targets’ safety and efficacy.
Parkinson’s Disease (PD)
PD is a common neurological disorder characterized by the loss of dopaminergic neurons in substantia nigra, causing a reduction in dopamine levels [4]. The best symptomatic treatment for PD was introduced in 1960 by the molecule levodopa. Besides this, there are many other treatments currently in use, like dopamine agonists, monoamine oxidase-B inhibitors, and catechol-O-methyl transferases (COMT), among others. Recently multitargeted compounds also experimented with for PD. Another approach that has been revealed to be useful in treating PD was deep brain stimulation (DBS). The surgically implanted electrode has an advantage of improving motor coordination but exacerbates the cognitive and emotional deterioration that erupts at the later PD stages [256].
Growth Factor Gene Therapy in PD
Considering gene therapy in PD, two strategic approaches can be made: symptomatic and neurorestorative approach. Symptomatic treatments include glutamic acid decarboxylase (GAD) and aromatic L-amino acid decarboxylase (AADC), whereas neurorestorative approaches involve NTFs, namely, neurturin (NTN) or GDNF, which are transferred via in vivo gene therapy [11].
NTN can bind to the GFRα 1 and 2 of the GDNF receptor and is a structural and functional homolog of GDNF [186]. NTN is a neuroprotective substance like that of GDNF [257]. Many restorative neurotrophins are known, out of which GDNF is seen promising in animal studies to mitigate PD pathology [258]. The transforming growth factor- β (TGF-β) members include GDNF and similar neurotrophins.
In preclinical and clinical studies, two GDNF delivery strategies have been studied, including AAV-dependent gene transfer and direct infusion of proteins [259]. The initially documented preclinical studies regarding GDNF gene therapy targeted substantia nigra as well as striatum of MPTP-lesioned and normal aged monkeys utilizing a LV vector [260]. AAV2-GDNF putamenal administration produced better clinical scores, raised DA turnover, and a raised uptake of PET-FMT in monkeys showing symptoms of PD for 6 months.
Apart from the AAV-mediated GAD and growth factor approach, several emerging ex vivo gene therapy approaches target PD. Transplantation of fetal NSC to MPTP-administered mice produced notable improvements in behavior regarding the protection of dopaminergic neurons [261]. The BDNF was increased in substantia nigra and striatum, and there was an increase of neurotrophin-3 and GDNF. There was a reduction of cytokines in substantia nigra and striatum and microglia in the striatum. The genetically modified human NPC generating GDNF was introduced into the striatum of rats with 6-OHDA lesions resulting in improved behavior and survival of dopaminergic cells. They showed neuroprotection in primates with MPTP lesion [17, 188].
The IGF-1 secreting modified NPC produced similar effects in rodent models [262]. The FGF-2 producing modified embryonic mesencephalic progenitor cells of the rat could not enhance the functions or survival of dopaminergic neurons in rats with 6-OHDA lesions showing every trophic factor does not show neuroprotective action [263].
In PD animal models, many researches have been done with NSC, which showed the protective activity for dopaminergic neurons showing the damage and enhanced behavioral recovery in striatum lesions. The MSC shows benefit because of immunomodulatory activities and growth factor production [264]. But MSC does not have evidence for long-term survival, limiting its therapeutic value in neurodegenerative disorders. The ex vivo gene therapy to deliver growth factors has not yet reached the clinics. The direct GDNF delivery targeting caudate-putamen showed potential in clinical studies [265, 266]. Many successful clinical and preclinical studies with direct delivery of growth factors and several successful ex vivo approaches employing cells producing growth factors, shows a promising future in the treatment of PD. A human NPC producing GDNF is being studied for ALS [15]. The expression of GDNF is an effective animal model for PD, and side effects that constitute the expression of GDNF include the downregulation of TH, aberrant axonal sprouting, and increased turnover of dopamine [267–269]. In another study, the E. coli dihydrofolate reductase with its destabilized domain (DD) fused with a LV vector was developed. Trimethoprim drug, which can cross BBB, binds and stabilizes the destabilized domain [270, 271]. It can stabilize the domain, expression, and extension of GDNF. This study is conducted in the rodent model before the induction of PD. Furthermore, studies are required to conduct on animals that already display PD symptoms [271].
The possibility of using gene therapy for the treatment of PD has been studied extensively and found safe and effective in phase I clinical trials, despite most of them have failed to show improvement in phase 2 trials, except for direct injection in the subthalamic nucleus by AAV2-GAD [72]. Another phase II clinical study of postmortem brain tissue found that NTN delivery to the putamen led to a rise in NTN I of the striatum/putamen, but not in the substantia nigra, that can be attributed to the failure of the retrograde transport of dopaminergic neurons [71, 272]. Another phase II trial was undertaken to resolve this transduction pattern, and NTN was injected into the putamen and substantia nigra. In such an approach, scientists hypothesized that gene therapy might be a success only when the growth factor is delivered before the neurodegeneration progresses extensively.
Miscellaneous Gene Therapy Approaches in PD
The AAV2-GAD delivers GAD with CED’s help to the subthalamic nucleus (STN) and inhibits local GABA, thereby pathological hyperactivity in basal ganglia. While AAV2-GAD strategy was examined in phase I and II trials, symptomatic amelioration was displayed to a lesser extent achieved by STN stimulation via deep brain stimulation or a neurostimulator [11, 187]. The gene therapy employing AADC is a prodrug strategy where the hAADC enzyme focal replacement produces an elevated transformation of levodopa to form dopamine and can be utilized in long term levodopa therapy. The therapy targets sensorimotor territory post commissional striatum. The striatum’s medium spiny neurons are not seen degenerating in PD, and research has shown these cells producing transgene expression for a larger time [273, 274]. The delivery of hAADC to promote with MPTP lesion in preclinical experiments resulted in notable better long term behavioral rating scores and notably reduced the requirement and adverse effects of levodopa [273–277]. The AAV2-hAADC delivery targeting putamen via iMRI-CED is being studied in a phase I trial aiming to track and modulate in real time the vector delivery with the help of iMRI-CED and can result in precisely calculating the vector coverage of the targeted brain areas. A two-center phase 1/2 open-label study of prosavin is done in 2014 [75], a LV vector encoding AADC and cyclohydrolase 1 tyrosine hydroxylase. No previous clinical trials employing LV vectors in humans have been reported. The therapeutic approach was to generate dopamine in a stable and continuous fashion in the motor areas of the putamen and showed safety in the first time use in humans, the LV vector–based gene therapy for the disease of CNS [11].
A clinical trial with prosavin targeting the dopamine synthesis pathway used trancistronic LV, with advantageous potential to deliver gene encoding for three enzymes, like GTP cyclohydrolase, tyrosine hydroxylase, dopa decarboxylase [75, 278]. The phase I and II trials were a dose-escalation study targeting the sensorimotor part of the striatum and putamen. As the main findings, the trial revealed success, with the drug showing a good safety profile and being promising in improving motor functions [69].
When a long-term phase I trial using a viral vector, AAV2 with AADC used as a transgene, an improvement in the PD rating scale was observed for the first year, but it was reduced for the following years [279]. The researchers focused on improving gene delivery by using an MRI-guided delivery system with more putamen coverage, anticipating AAV2-AADC to be a better therapy in humans [280]. The expression of tyrosine hydroxylase (TH) and GTP cyclohydrolase 1 (GCH1) to synthesize dopamine has experimented in non-human primate models of humans and rats using the AAV5 vector. The rat gave a promising result, but in primate models failed to express TH [281, 282]. A preliminary rat model with two groups was taken to assess cerebral dopamine NTF efficiency, with both reporting functional improvements and only one TH protection [283, 284]. Achieving control over the transgene expression may refine gene therapy as sometimes transgene, and its continuous expression may lead to several side effects.
Amyotrophic Lateral Sclerosis (ALS)
ALS is a progressive neurological disease known as Leu Gehrig’s disease that causes weakness of respiratory muscles, arms, and legs, sporadic or familial presentation, and fatal within 2–5 years [4, 285, 286]. The familial ALS can be approached in gene therapy by specific mutations, whereas in sporadic ALS, the neuron is supported by the delivery of NTFs [285, 286].
Growth Factor Gene Therapy in ALS
Aebischer et al. performed phase I clinical trial of encapsulated genetically modified xenogeneic cells in ALS patients via intrathecal route following initial systemic delivery of CNTF and concluded persistent delivery of neurotrophic factors with human CSF via ex vivo approach can open doors for further treatment of neurological diseases including ALS [287]. Team effort by Suzuki and colleagues reported the GDNF expressing MSCs engraftment onto rat skeletal muscles could spar death of motor neuron death and ameliorate survival directing towards a practical ex vivo gene therapy approach in ALS [288–290]. Another effort by Acsadi et al. to study AV-mediated GDNF expression in transgenic neonatal SOD1 mice muscles demonstrated prolonged survival and a delay in motor function decline which also opens up chances of GDNF in ALS treatment if successful in clinical trials [291]. Neurotrophin-3 (NT-3) delivery via AV vector can also be an option in motor neuron diseases including ALS but lacks bioavailability and risk of toxicity which recommends this approach to be of a lower choice in clinical practice [292]. Another approach is AAV-mediated IGF-1 delivery into mice muscles that could offer delayed onset of disease as well as neuroprotection. But the rationale in selection of both vectors and growth factors matters that could yield successful results [293].
Miscellaneous Gene Therapy Approaches in ALS
In ALS, earlier studies of cell-based therapies focused on replacing motor neurons [294–297]. Other approaches focused on trophic factor production, thereby improving motor nervous and motor functions [298–300]. Studies were conducted with modified NPC and NSC showing growth factor overexpression and its transplantation improved motor neurons’ survival. Research conducted with NSC producing GDNF and IGF-1 resulted in improved motor neuron survival in the ALS animal model, whereas NSC that express VEGF, NT3, and BDNF could not produce results [15, 301]. In many studies utilizing the SOD1G93A ALS rat model, the NPC, which can express GDNF administered via intraparenchymal injection, had remarkable motor neurons [18, 196, 302]. Still, the locomotive function seems unaffected as there is no protection of the muscle innervation. A recent clinical trial administers modified NPC that can express GDNF to the lumbar spinal cord of patients with ALS and is the first ex vivo gene therapy with neural cell researched in humans to treat neurodegenerative disorders. Treatment with MSC delivered via several routes showed beneficial effects in animal models of ALS [303]. Various studies showed that intrathecal and intramuscular delivery of MSC-NTF is beneficial and relatively safe for ALS treatment [304]. MSC expressing GDNF showed benefit in ALS pathology, and MSC expressing VEGF showed similar activity [197]. Several ex vivo gene therapies have been studied in animal models of ALS with varying results.
A major hurdle in research is the unavailability of sporadic ALS animal models and delivery techniques for studying potential therapies [15]. One of the gene therapy trials found to be effective was superoxide dismutase 1 (SOD1), where drifts in SOD1 cause ALS. The safety profile of this treatment was established by administering antisense oligonucleotides to SOD1 intrathecally. Another study involved AAV9-SOD1-Sh RNA in opening up SOD1 gene expression in rat motor cortex, and the injection was given via tail vein or by temporal vein [286, 305]. Both trials exhibited extended survival and delayed disease inception. Another study that can transfer to the clinic is a non-human primate trial, which was successful in intrathecal delivery and SOD1 suppression [306]. This trial revealed a delayed disease onset and an extended life span in transgenic mice, although it restricts only to SOD1, so that it can extend up to the other mutations causing ALS.
A neuroprotective approach has also been adopted for sporadic ALS, achieved by motor neuron protection through growth factors delivery. The worth of note is that promising results have been achieved at preclinical level but not at clinical one.
Also, vascular endothelial growth factor (VEGF) has been linked to ALS, where VEGF deletion in the promotor region exhibit neurological disorders similar to SOD1 [200, 307]. Of VEGF expression studies conducted in animals, two of them were AAV4- IGF1 and AAV4-VEGF [200]. They are delivered into the lateral and ventricles of SOD1 mice and found to improve the extended survival and delayed onset of the disease. Another approach was the expression of a protein called zinc finger by delivery of plasmid which might increase the expression of VEGF. This study was conducted in SOD1 rats with weekly 8 injections gastrocnemius muscle which showed refinement in motor functions, but not in overall weight or life span [308–311]. The only growth factor which showed improvement was granulocyte colony-stimulating factor (GCSF) when injected into the spinal cord of SOD1 mice [312, 313].
Another potential source of transgene involved in the regulation of mRNA expression is enzyme adenosine deaminase acting on RNA 2 [ADAR2] and up frames shift protein 1 [UPF1] [312, 313].
Spinal Muscular Atrophy (SMA)
SMA is a childhood neurological disease caused by the loss of survival motor neuron 1 (SMN1), which leads to infantile death [314]. SMA is ideal for gene therapy as only one gene is responsible for the disease [315]. Replacement of SMN1 showed efficacy in mice studies, where researchers used the route of administration of AAV9 as both intrathecal and intravenous to take over the expression of SMN1 in the spinal cord [202–204, 316]. In a phase 1 trial of the AAV9-SMN1 study initiated in 2014, safety and efficacy investigations continue to be performed. Similarly, studies in pig models of SMA have also focused on the SMA adversity and intervention mediation, where the disease is induced by the knockdown of SMA1 expression [317]. Most animal studies implicate a delay in disease progression, but only the partial progression in motor neuron numbers. A recent study on AAV vectors’ engineering states that gene delivery via AAV vector is approbated for SMA treatment [61].
Spinocerebellar Ataxia
Spinocerebellar ataxia (SCA) is a neurodegenerative disorder caused by cytosine adenine guanine (CAG) repeats in ataxin-3 gene resulting in dysfunction cerebellum and brainstem. Another type of SCA is SCA3, known as Machado-Joseph disease (MJD), an autosomal neurodegenerative disorder with rare occurrence, whereby the accumulation of mutated ataxin-3 protein causes protein misfolding [4]. SCA1 and SCA7 are similarly targeted through direct brain injection, with results indicating that RNAi is a promising tool for treating many SCA forms. SCA1 can be targeted by RNAi, which suppressed neurodegeneration induced by polyglutamine in the SCA1 mouse model. Suppression of Atxn3 in SCA3 rats via LV delivery demonstrated mitigation of degeneration but required further preclinical confirmations. Similarly, miRNA delivery in SCA3 mice improved molecular phenotype but still requires further preclinical replication. Antisense oligonucleotide ASO) approach targeting against ATXN1, ATXN2, and ATXN3 demonstrated a reduction in these protein levels, but further preclinical confirmation is required, and no clinical observations have been reported so far. The usage of miR-3191–5p in SCA6 animal models demonstrated protection against Purkinje cell degeneration, motor deficits, and ataxia but required strong preclinical as well as clinical studies. Direct delivery of miRNA or AAV in SCA7 mice cerebellum showed an improvement in ataxia phenotype, but there is a need for strong preclinical and clinical observations [205]. Another approach is small interfering RNAs (siRNAs) in SCA3/MJD transgenic mouse models targeting mutant ATXN3, which were encapsulated within stable nucleic acid lipid particle (SNALPs) delivering selective and effective silencing with improvement in neuropathology and motor behavior [318].
In an open-labeled study, IGF-1 has been tested in SCA3 and SCA7 resulting in a decreased scale for assessment rating of ataxia (SARA) score and treatment using insulin-like growth factor-1/insulin-like growth factor-binding protein-3 (IGF-1/IGFBP-3) showed higher expression level in SCA3 subjects resulting in an increase in an autophagy-mediated protection [319, 320].
Neuropeptide Y(NYP) is under-expressed in MJD mouse models and patients, and parenteral administration of NYP via AAV vector could reduce the accumulation of ataxin-3 in mouse models, while improve BDNF expression and motor coordination, and preserves granular layer thickness and cerebellar volume [320]. For example, the intramuscular administration of NGF in SCA3 patients for 28 days led to a decrease in SARA score, improvement in speech, finger chase, stance, heel-shin slide, and hand movements [320].
Epilepsy
Epilepsy is a disease characterized by neuronal excitability occasionally and sometimes as unpredictable seizures [321]. Seizures can be of different types, like in generalized form, bilaterally distributed network and in focal, and distributed to only one brain hemisphere. Gene therapy can be a good alternative therapy for epilepsy caused by lesions. A study by incorporating HSV vector containing transgene encoding two NTFs, fibroblast growth factor 2, brain-derived NTFs are injected into the lesion area which can cure the cell loss and reduce the epileptogenesis [206–208]. Another approach is to rearrange the glutamate or GABA receptor composition to modulate receptors’ response, which gave promising results in epileptic animal models [322]. Later another propitious result was given by the delivery of vectors containing the transgenes of NYP, which resulted in consistent antiseizure effects that can be applied clinically [209, 210, 323]. A patient with surgical resection of the epileptical region is an ideal candidate for gene therapy. Seizure inhibitory factors like NYP can be transferred along with depth electrodes to know the necessity for surgical procedures to transfer the vector. If gene therapy fails, the patient can go ahead with surgical intervention as planned [211, 324–328]. Another approach to reducing seizure frequencies can be by the restoration of neuropeptide balance. One such is CG01, an amalgam of NPY and Y2 genes delivered into the brain post-surgery confirmed antiepileptic effects. Kullmann and team’s serendipitous observation encouraged them to develop a modified Kv1.1 gene introduced into a viral vector specifically targeting the excitatory neurons. Both Kv1.1 and CG01 have prolonged duration action at a single injection, which can be due to the regulated production of growth factors or ion channel functioning. In contrast to Kv1.1 and CG01, studies on GluCl and the regulation of membrane proteins such as opsins capable of keeping neurons inert have been done on nematodes, giving a disadvantage of immune chances system rejection [213, 329]. Additionally, in the case of epilepsy that is drug-resistant, investigations using LV gene therapy are performed in phase I and II trials [330].
Huntington’s Disease (HD)
Huntington’s disease is produced by excessive CAG trinucleotide repeats on chromosome 4 exon 1 and is an autosomal dominant disorder and results in polyglutaminated huntingtin (mHtt) protein [331]. The raised mHtt aggregate levels in pyramidal cortical neurons and medium spring neurons of the striatum result in a pathological phenotype seen in HD. The protein mHtt generates energy dysregulation by restraining the PGC-1α action, which is essential for mitochondrial function and prevents individual neuronal volume [331]. A regulatory protein, calmodulin (CaM), plays a significant role in regulating the calcium by activating many enzymes for calcium-binding, and HD patients can show aberrant calcium signaling in the cytoplasm [332].
Growth Factor Gene Therapy in HD
NTFs, like ciliary neurotropic factors (CNTF), BDNF, and fibroblast growth factors (FGF), also can enhance the neuron lifespan [217, 218, 333]. Research with NPC, NSC, and MSC transplanted into HD animal models produced improvement of function, showing ex vivo gene therapy’s potential in treating HD [334]. These modified cells express trophic factors, which seem beneficial in HD. In a study, NPC/NSC expressing GDNF are transplanted and resulted in better survival of neurons in the striatum and produced a reduction in behavioral deficits associated with lesions [15, 219, 220]. Several studies utilizing ex vivo gene therapy for HD have been focused on modified cells expressing BDNF. But the drawbacks in methodology and limited results alternate concepts are in need [225, 335]. The ex vivo gene therapy transplanting cells that express molecules modulating astrocyte and microglial function need to be studied for their potential benefit in HD therapy [15].
Miscellaneous Gene Therapy Approaches in HD
Gene therapy in HD can directly target the nuclei showing dysfunction, thus lowering mHtt levels. The gene therapy approach in preclinical studies utilizes RNA interference (RNAi) to reduce abnormal mHtt volume. A study utilized AAV5 with a transgene encoding delivery, an engineered miRNA towards HTT mRNA [214–216]. The AAV5 delivery resulted in wide transgene distribution [336] and is ideal for the therapy of HD [11]. Studies on a recombinant antibody that can reduce the mHtt aggregation have also been done [337].
Stroke
Transforming growth factor β (TGFβ) secreted by the astrocytes can cause capillary endothelial downregulation resulting in a stroke, which can be either ischemic or hemorrhagic [4]. Gene therapy can help treat ischemic and hemorrhagic stroke due to its ability to stabilize blood vessels. Receptors involved in treating stroke include C-X-C chemokine receptor 4 (CXCR4) and neuronal death are prevented by HSP72, GDNF, and B-lymphoma 2 [BCL2] [224, 226, 338].
Growth Factor Gene Therapy in Stroke
NSC expressing GDNF/BDNF post-infarct administration produced slight behavioral betterment in an animal model of stroke [15, 339, 340]. A reduction in the infarct and improvement of neurological deficits were observed in the cerebral ischemia rat model after administering modified MSC expressing VEGF, GDNF, and BDNF [341–343]. Various preclinical research utilizing stem cells produced promising results, which can be due to improved plasticity and survival of neurons because of the molecules expressed by the stem cells transplanted [344, 345]. The clinical translation showed safety, but the effectiveness is not yet confirmed [345, 346]. A useful therapy dependent on stem cells for stroke needs an ideal therapeutic window. The stem cells that can survive in post stroke areas should be able to deliver molecules that improve synaptic plasticity and neurogenesis and modulate neuroinflammation while providing trophic support [15]. Another approach is FGF-2 delivery via AV vector performed on Mongolian gerbils found to promote neurogenesis post cerebral ischemia compared to direct administration of FGF-2 due to an increase in the proliferation of progenitor cell [223].
Miscellaneous Gene Therapy Approaches in Stroke
Reperfusion therapy to restore the blood flow may cause brain injury, production of harmful oxygen-free radicals. The generation of the radical can be subsided by gene therapy by the delivery of genetic materials that can eliminate the free radicals (heme oxygenase-1 [HO-1], glutathione peroxidase [Gpx], and superoxide dismutase [SOD]. The glucose uptake of neurons is influenced by the glucose transporter gene (GLUT-1), which hinders the energy reduction, which induces cell death [227, 347, 348]. The ex vivo gene therapy strategies targeting stroke seems beneficial in neurological repair. Modified NSC expressing galectin-1 was studied, focusing on neuroprotection and modulation of neuroinflammation [15, 349]. The NSC, after administration, were found surviving and produced a notable reduction in tissue loss, and enhanced cognitive and sensorimotor functions were seen with cells expressing galectin-1, which showed promise in vitro studies improving the microglial generation of IL-10, TGF-β neuroprotective as well as anti-inflammatory cytokines and produced and inhibition of pro-inflammatory mediators. Another strategy produced a slight improvement in infarct and behavior size after the administration of modified NSC secreting copper/zinc superoxide dismutase (SOD1) [15, 350].
Spinal Cord Injury (SCI)
SCI’s mechanism is necrosis of neurons and secondary mechanism includes ischemia, inflammation, and delayed apoptosis, which follow and worsen the functional losses. Emergence of CRISPR/Cas9 genome editing, enabled the possibility of gene editing in extenuating the adverse effects caused by SCI. Mainly two significant applications for SCI amelioration via gene therapy approach are known, and these include either ex vivo cell transduction or in vivo delivery directly to spinal cord by non-integrating AAV. Preclinical investigations on spinal cord injury could reveal in vivo gene therapy applications for enhancement of pro-regenerative factor expression, gene silencing of inhibitory factors, neural circuit modifying factors, and inhibitory particle degradation by enzymes that modify matrix [229]. Enhancement of pro-regenerative factors such as Kruppel-like factors (KLFs) and SOX11 promotes axonal regeneration and neurogenesis. Short-hairpin RNA (shRNA) are capable of silencing inhibitory factors say, for example, a tumor suppressor, phosphatase, and tensin homolog (PTEN) via mTOR downregulation can reduce damaged neuron regeneration. Using staggered double hemisection (SDH)-SCI studies, reformation of bypass circuits surrounding the lesions or damaged neurons is possible. Through SDH model in mice, chloride potassium symporter 5 (KCC2) was identified which could maintain a balance in excitatory and inhibitory neurotransmission ratio via modulation of neural circuits and AAV mediated overexpression of KCC2 could improve the functional recovery with less or no adverse effects by influencing synapsin promoter. Preclinical studies using chondroitinase ABC (ChABC), a bacterial enzyme reveals that enzymatic degradation of the secretions from glial scars such as CSPGs can improve neuronal functionality and regeneration. Application of gene therapy that is by incorporating LV vector to ChABC gene resulted in a long-term expression the gene that could improve fine motor recovery post cervical SCI [229]. For the regenerative treatment of SCI and to refine the axonal regrowth in SCI patients, several therapeutic genes have been tested, like NGF, GDNF, neurotrophin-3, decorin (DCN), CNTF, galectin-1, and neural cell adhesion molecule (Ncam1) [351–354]. DCN suppresses inhibitory chondroitin sulfate proteoglycan, which will improve the axonal regrowth of neurons. Growth factor α can target astrocytes and can increase its invasion towards the damaged neural cells and improves axonal growth into lesions. Remyelination of oligodendrocytes is essential to stabilize the axons, and necessary myelin protein (MBP) and leukemia inhibitory factor (LIF) are involved for the purpose. Various therapeutic genes support secondary injuries, regrowth, neuroprotection, remyelination, and improving the microenvironments in spinal cord injury. Some of them are collagen type 4-α1, laminin subunit-α, heparin sulfate proteoglycan 2, and fibronectin 1 [355–360].
Traumatic Brain Injuries
Traumatic brain injuries (TBI) are complex brain injuries that are progressive as a result of neuroinflammation and other physiopathological mechanisms, and no treatment strategies are approved so far. TBI can be categorized based on symptoms, etiology, prognosis, and anatomical abnormality. Classification based on clinical features consists of mild, moderate, and severe TBI. Pathoanatomical classification of TBI includes contusions, diffuse axonal injury, hematomas (epidural, parenchymal, and subdural lesions are included), and subarachnoid hemorrhage (SAH) [361]. Mild TBI/concussions occur as a result of non-penetrating trauma on the head and develop symptoms that not only include behavioral and cognitive defects but also physical symptoms such as dizziness, nausea, and vomiting. Being of less severity, about 90% of mild TBI resolves in a week time or about 12 weeks in case of post-concussive patients. Unlike mild, both moderate and severe forms of TBI are concerns of intensive care and/or neurosurgery. Moderate TBI can last for months with behavioral, cognitive, and other physical complications. Severe TBI are life-threatening which might have developed as a result of penetrating injuries capable of damaging dura [362].
Gene therapy has been advancing in the area of TBI as studies reveal role of specific genes in causation of the disease. TBI is known to express genes both that are self-destructing and protective genes. Hence, targeting on specific genes responsible for the pathogenesis of TBI can block the cell death and aid in self-repair of the neurons [231]. Gene level studies in TBI include determining the expression of the NTF (NGF), proapoptotic, and anti-apoptosis proteins in Bcl-2 family, heat shock protein (HSP-60, 72, 32), and zinc finger protein (A20). Additionally, there are genes that are targeting in ameliorating neuronal death post TBI and this includes GLUT1 which is responsible in the inhibition of necrosis associated factors. However, the targeted delivery of genes requires appropriate vectors. AV delivery of genes were found to be robust compared to other viral and non-vectors. AV delivery of GDNF can attenuate focal cortex trauma and expression of Bcl-2 fusion protein using recombinant AV could prove to be neuroprotective post TBI.
Potentiating pro-inflammatory vectors can cause progression of injury which can be evaluated using suitable TBI models [230]. Elevation of tumor necrosis factor alpha (TNF-α), elevated c-fos expression, and HSP-72 has been observed in animal fluids post TBI indicating that the expression of these genes has a critical role in TBI, whereas expression of NGF, proteins from Bcl-2 family, and HSP-70 are decreased in TBI or showed features such as promotion of growth, survival, and differentiation of neuronal cells [231]. By the incorporation of viral vectors and certain non-viral vectors to growth factors, there was an attenuation in traumatic injury in focal cortex and these include GDNF-adenoviral delivery, gene transfer via AAV8, transfer of calbindin D gene via HSV, NGF expression via transferrin-associated cationic liposome/Tf-lipoplexes.
However, there are a lot of obstacles involved in practicing gene therapy in TBI. Biosafety is a concern in use of viral vectors, and the gene therapy approach has a lot of compatibility issues which is not favorable in clinical practice. For example, unlike animals, direct intracranial administration is not preferable whereas intravascular approach in clinical set up has drawbacks such as low BBB permeability. Another issue is with the targeting of specific genes as about 200 genes are expressed and interact among each other forming a regulative network with much complexity [231].
Canavan Disease
Canavan disease (CD) is a rare genetic, progressive metabolic neurological disorder belonging to the leukodystrophies category, occurring mainly in infants due to aspartoacylase (ASPA) mutation. Initially infants appear asymptomatic for a period of 2–3 months and gradually develop macrocephaly, reduced head control, floppiness, and delayed developmental milestones. Both viral and non-viral vector-mediated gene therapy approaches have been under clinical development and show a practical safety profile, despite having limited clinical benefits. AAV-mediated ASPA gene delivery has been conducted in phase 1 clinical trial with 13 patients, whereas non-viral vector and liposome-mediated ASPA delivery has been underwent in 2 patients [363].
Lysosomal Storage Disorders
Lysosomal storage disorders (LSDs) are rare genetic metabolic disorders occurring due to defect in the functioning of lysosomes, including autophagy impairment, vesicle trafficking aberration, mitochondrial dysfunction, dysregulation of various signaling pathways, and calcium dyshomeostasis. Patient-derived iPSCs are capable of addressing these defects in lysosomal functioning; however, the genetic diversity causes an inconvenience in proceeding this technique. An amalgam of CRISPR-Cas-9 editing and iPSC can be utilized for the novel pathway identification and screening of drugs for LSDs therapy. Moreover, CRISPR-Cas-9 editing can be used for altering mutants responsible for LSDs. In vivo LSDs models have been developed using gene knockin and knockout technologies. So far, both in vivo and ex vivo approaches have been used in LSDs gene therapy, with the in vivo approach involving transduction of organ-specific or ubiquitous regulated transgene via AAV vector, whereas ex vivo approach involves hematopoietic stem cells subjected to genetic modification and re-implantation using lentiviral vectors. Another strategy is antisense oligonucleotides utilization found to have good impact on larger portion of patients [364].
X-Linked Adrenoleukodystrophy
X-linked adrenoleukodystrophy (X-ALD) is a rare genetic peroxisomal neurodegenerative disorder affecting adrenal glands and nervous system which can range from a severe cerebral defect in childhood to a delayed onset of adrenomyeloneuropathy (AMN) with a higher incidence in males due ABCD1 gene mutation which is supposed to encode adrenoleukodystrophy protein (ALDP), whose defect can cause saturated very long-chain fatty acids accumulation. Studies have shown that long-term lentiviral-mediated hematopoietic stem cells (HSCs) gene therapy can arrest the devastative progression of the disease. Slow progression of axonopathy is the hallmark of X-ALD and this feature needs to be addressed and studies on Abcd1/Abcd2 double-deficient mice models have been conducted by evaluating the potential of neurotrophic factors, such as NT-3, and IGF-1. The mice treated with AAV-6 mediated NT-3 or IGF-1 showed a marked improvement in motor behavior, axonal maintenance, oligodendroglial support, and neuronal integrity for AMN [365–368].
Rett Syndrome
Rett syndrome (RTT) is a rare genetic neurodevelopmental disorder affecting the brain in progression of speech and motor skills with a higher incidence in female babies due to X-linked gene, MECP2 mutation. Studies on mouse models have been conducted by silencing MECP2 by lox-stop cassette insertion, causing a reduction in the mortality as well as improvement in neurological signs. Another approach is the overexpression of BDNF in MeCP2-mutant mice which could reverse locomotor defects, despite limitations for the application of gene therapy in RTT; for example, it is required that MeCP2 transgene be expressed in mutant cells thereby avoiding its overexpression in the healthy allele. Lentiviral-mediated MECP2 delivery has yielded a reversal in phenotypic maturation of dendritic cells which needs to be proceeded for in vivo studies. IGF-1 is another growth factor regulating plasticity and synaptic maturation, and inhibition of this growth factor signaling could regulate aberrant levels of MECP2 due to the presence of a binding site in RTT patients and MeCP2-null mice [369]. Based on a previous study demonstrating restoration of synaptic molecules in Mecp2 KO mice by the administration of IGF, Castro and team made use of recombinant human IGF1 (rhIGF1) in symptomatic heterozygous female as well as Mecp2-null male mice. This study aligns with the previous studies and clinical trials with regard to the efficacy and safety of rhIGF1 in RTT treatment and the team could observe an improvement in behavior, synaptic plasticity, and activation of cellular pathway [370]. A novel gene therapy candidate for RTT has been developed by Novartis gene therapies by the name AVXS-201 which is in queue for USFDA approval for proceeding into clinical trials. AVXS-201 is delivered via AAV9 where by MECP2 protein will be encoded optimally and compensate for the defective mutated MECP2 [371].
MicroRNA-Based Gene Therapy
About 30% human genes are regulated by non-coding RNAs called microRNA (miRNA) which control various cell processes and gene expression. CNS and metabolic disorders can occur as a result of miRNA dysregulation. miRNA can be approached as either diminishing disease induced miRNA expression or replacing disease repressed miRNA expression. miRNA can downregulate different targets as well as enables delivery by multiple drug delivery systems, but has a low precision rate. In AD, miR-16 activation via liposomal form is targeted in HEK293 cells for reducing BACE1 and APP expression. Liposomal-miR-16, 15, and 195 activates both neuro2a and HTT2 cells thereby reducing BACE1 and APP expression. miR-132- oligonucleotide potentiates ITPKB in mice ameliorating amyloidosis and τ-hyperphosphorylation. In PD, miR-155 via striatal injection in microglial cells could regulate the α-synuclein associated inflammatory responses. Liposomal miR-96 acts as an inhibitor of CACNG5 in SH-SY5Y cells causing elevation of nigral cells. In HD, miR-27a stimulates MDR-1 and reduces accumulation of mHTT in derived neuronal stem cells. miR-196a activates HTT in human embryonic renal cells and neuroblastoma cells of mice and cause a decrease in mHTT expression. In ALS, Src homology-2domain-containing SHIP1 and suppressor of cytokine signaling-1 is inhibited by miR-155 administrated via intracerebroventricular route in SOD1G93Amice leading to prolongation in survival rate and slowing down disease progression. A single miRNA targets multiple pathways simultaneously offering chances of toxicity. Another limitation of miRNA system can be sex-biased and age-influenced. Hence, therapeutic strategies must be developed sex specific and patient monitoring post miRNA therapy application will be required [3, 372].
CRISPR-Cas System and Other Gene Editing Technology
Complementary to gene therapy strategies, genome editing has emerged with an aim to regulate or repair endogenous gene or else inactivate the toxic gene. However, this approach lacked efficient delivery systems, production being complex and limited efficacy kept them in halt from clinical application in the past few decades. The advancement in precise and sophisticated genome editing tools has paved the way in drug discovery, genetic diagnosis, and even basic biology techniques which were all far approachable. The key features in editing involves recognition of a unique target sequence within the DNA-binding domain and secondly to induce precise modification in genetic or epigenetic level using an effector element [373].
CRISPR-Cas System
Novel gene editing approaches can be utilized in the treatment of neurological disorders which are far more efficient, stable, and fast compared to the conventional treatment and diagnostic methods and could be beneficial in advancement of gene therapy in neurodegenerative diseases. Gene editing is accurate and involves targeted cleavage of specific double stranded sequence of the DNA and utilization of various DNA repair pathways. Cleavage is mainly performed by nucleases namely, ZFN, TALEN, meganucleases, and CRISPR-Cas-9 system [374]. CRISPR is gaining a lot of attention these days due to lacunae in effective treatment of neurological disorders.
Aβ deposition is considered the hallmark of AD neuropathology. In addition to this are other targets such as γ-secretase protease, APPswe APP 3′-UTR deletion, apolipoprotein E4 (APOE4) allele, and glia maturation factor (GMF) which also requires attention in alleviating AD. Aβ deposition being the major cause in AD development can be targeted by either overexpressing enzymes capable of degrading Aβ or anti-Aβ antibodies. An investigation by Hanseul Park and team in suppressing β-secretase 1 (Bace1) gene showed Aβ-associated pathological inhibition [374, 375]. Wong. E. et al. worked on GSAP gene knockout in HEK293 cell lines and found that this had direct association with γ-secretase activity decrease which in turn caused reduction in Aβ generation [374, 376, 377]. Another study consisting of in vivo as well as ex vivo by György et al., on the mutation of APPswe by the application of CRISPR/Cas9 using Streptococcus pyogenes revealed that disruption in APPswe lead to fall in Aβ level [374, 378]. Another evaluation by Nagata et al. in editing APP gene by deletion of 3′-UTR confirmed fall in APP expression which mitigated Aβ accumulation in the brain [374, 379]. A definite risk of AD by APOE4 allele varies with prevalence of SNP and studies on iPSCs of E4 allele carrying AD patients were edited to E3/E3 genotype. It was observed that E4 unedited neurons caused elevation in τ phosphorylation [374, 380, 381]. GMF, a proinflammatory molecule when edited using CRISPR/Cas9, eliminated microglial activation and suppressed MAPK that are usually found upregulated in AD [374, 382].
α-Synuclein is a definite pathology of PD and is encoded by SCNA gene which is overexpressed in the presence of a common variant [383]. Another target that is involved in inducing dopaminergic neuron toxicity for sporadic PD include leucine-rich repeat kinase 2 (LRRK2) that induce toxicity in the dopaminergic neuron which when edited using CRISPR/Cas9 tool showed reduction in the incidence of sporadic PD [384]. p.G2019S is the most common mutations found among LRRK2 gene. Editing of LRRK2-G2019S in hiPSCs and utilizing technologies such as piggyBac transposase revealed reduction in tyrosine hydroxylase (TH) positive neurons which in turn causes reduction in LRRK2-G2019S dopaminergic neurons [384]. Another approach to PD pathogenesis is targeting the neuroinflammatory pathway which includes PKCδ expression knockdown within the dopaminergic neurons causing an obstruction of DNA fragmentation which are Mn-induced [385]. A study by Gordon et al. on prokineticin 2 (PK2) elimination via prokineticin receptor 2 along with CRISPR/Cas9 demonstrated enhanced chances of cell death stimulated by neurotoxicant [386]. In an investigation done by Chen et al., hESCs deletion using CRISPR/Cas9 and further inclusion of recombinant α-synuclein pre-formed fibrils (PFFs), wild-type neurons demonstrated susceptibility to pS129-αSyn-positive protein gathering indicating Lewy-like pathology observed in PD [387]. Selvakumar et al. worked on establishing reduction in pro-inflammatory mediators within the brain as well as microglial activation by knockout of microglial GMF using CRISPR/Cas9 technique. This was achieved by a number of mechanisms involved including lowering of ROS production as well as calcium flux thereby weakening oxidative stress. In addition to this, NRF2 nuclear translocation also decreased. Hence, a reduced expression of cyclooxygenase 2 (COX2), nitric oxide synthase 2 (NOS2), HO-1, and ferritin activation can occur in the microglial cells [388].
Unlike AD and PD, HD does not have a certain cure so far and abnormal HTT protein is the pathology behind the disease progress. Hence, gene therapy can be promising in the treatment of HD which is currently investigated by targeting DNA transcription or decreasing RNA translation thereby reducing abnormal HTT protein level [389]. Two approaches in gene therapy, zinc finger proteins and CRISPR/Cas9 via direct DNA interaction, can suppress mHTT gene expression [390]. In one study, CRISPR/Cas9 is known to effectively reduce endogenous mHTT production in striatum, whereas in another study identification of SNPs has gone through a different approach whereby Cas9 nuclease efficiency is enhanced and CRISPR focusses on therapeutic strategy [375, 391]. mHTT presence can elevate entry of neuronal store-operated calcium (nSOC) and outflow of calcium from ER leading to InsP3R1 sensitization. Recent studies on the inhibition of transient receptor potential canonical 1 (TRPC1), a component of nSOC, proved to improve motor performance in both in vitro and in vivo and hence can have a neuroprotective potential in patients if investigated further in clinical trials [392]. Another probable target in HD can be 5′ untranslated region (UTR) which plays a crucial role in the regulation of HTT protein synthesis. 5′ UTR consists of upstream open reading frame (uORF) which is capable of regulating downstream ORF translation. uORF disruption using CRISPR/Cas9 can reduce mHTT translation within MSCs [393]. Kolli and team through their study found that mHTT silencing by using CRISPR/Cas9 system could work in either way, either by nicking DNA at the boundary of exon1-intron or by decreasing mHTT production in bone marrow–derived MSCs. CRISPR/Cas9 system has accuracy in diagnosis and disease detection, however have limitations in HD-associated regions due to detection of high guanine-cytosine content; hence, detection methods needs to be improved [394].
Other Genome Editing Approaches
A permanent alteration or transient modification of the DNA sequence can be achieved by base or gene editing for the former and epigenetic editing or gene regulation for the later. Gene editing causes permanent alteration in DNA sequence by insertion, deletion, or substitution via homology-directed repair (HDR) pathways which are precise but less efficient than non-homologous end-joining (NHEJ). The effector domain induces double-strand breaks (DSBs) at the DNA sequence target which is to be modified. However, gene editing has limitations which include chromosomal instability, chances of off-target cleavage, target sequence restriction as in PAM for CRISPR and 5’-T for TALENS, and heterogeneity in NHEJ. Unlike gene editing, base editing does not induce DSBs, thereby lessening the possibility of off-target insertions and deletions. This platform is involved in the modification of single nucleotides and can be applied for correcting point mutations observed in human genetic disorders. The shortcomings are that this platform lacks efficiency and product purity; there are only possibility of substitution within the sequence, RNA and DNA level off-target cleavage, and bystander base editing [373]. Genome regulation can be achieved either by epigenome editing or transcriptional modulation. Epigenetic editing of the epigenetic marks is a long-term modification and enables cell reprogramming. However, information regarding epigenetic marks for some targeted genes are yet not available. Several epigenetic marks will have to be modified simultaneously and this might affect a large genome region. Transcriptional regulation enables cell reprogramming with low off-target effects and expression at physiological level. Hence, the efficacy is gene expression level dependent and permanent modifications are not achieved. This platform also affects a large genomic area [373].
Current Challenges in Gene Therapy
There are several limitations currently faced in gene therapy which affects its safety and efficacy. There are barriers such as biological barriers as well as immune responses, rate limiting steps in the expression of transgene based on cell type, and possible undesirable effects associated with vectors or treatment strategies. Overcoming these barriers and limitations greatly enhances the benefits of gene therapy.
The current therapeutic strategies utilize alternative routes, alternative serotypes, as well as alternative clinical compounds [395] redirection or expansion of vector tropism by modifying viral capsid including chemical modification such as PEGylation, inclusion of cell penetrating peptides and epitopes, utilizing capsid variant, chimeric, hybrid, mosaic, and capsid decoys vectors to overcome biological as well as physical barriers [395–397].
Higher doses of vector, alternative routes, alternative serotypes, utilizing proteasome inhibitors, plasmapheresis, host immunosuppression, and developing inert vectors by modifying viral capsid, are being employed to overcome immune responses [395–397]. Dual or single self-complementary AAV that does not need host DNA synthesis, hybrid vectors, and proteasome inhibitors are being employed to overcome transgene expression rate limiting steps. Also, tissue-specific promoters, hybrid vectors or trans-splicing, replication defective vectors, non-infectious virus, like particles, exosomes, vexosomes, artificial chromosomes, and chromatin insulators are being employed to overcome undesirable effects of vectors.
Even though these approaches improved gene therapy in preclinical studies, they are yet to provide benefits to the patients. Several novel approaches in gene therapy are being studied and developed and can further improve gene therapy in future. Developments in tissue engineering technologies are providing various new options to deliver therapeutic compounds. The biomaterials utilized includes natural as well as synthetic and can be manipulated for delivering therapeutic compounds [398].
The novel possibility of controlling gene vector delivery can greatly improve the safety and efficacy of gene therapy. The controlled release strategy can improve residence time of therapeutic compound in the targeted tissues, protect viral capsid epitopes from host immune reaction, help deliver larger genes, minimize vector doses, and improve stability. Gene vectors can be immobilized or encapsulated for a substrate mediated or gradient polymeric release.
Delivery strategies such as solid scaffolds provide transfer of vector from the substrate, and hydrogels providing vector diffusion are being studied for gene therapy. Solid scaffolds including sponge, membranes, minipellets, and disks are being developed utilizing collagen and can target the skin, muscles, and bones. Alginate as well as gelatin scaffolds can be utilized for controlled delivery of viral as well as non-viral vectors targeting tumors, cartilage, and also as vaccines. Polyethylene glycol (PEG) sponges with LV vectors have been studied for spinal cord delivery. PLGA scaffolds and poly(lactide-co-glycolide) (PLG) gene delivery can deliver viral as well as non-viral vectors in the spinal cord, muscles, skin, brain, blood vessels, as well as bone, for vaccination as well as anti-inflammatory treatment. Polyurethane scaffolds are studied to deliver viral vectors for wound treatment and also to the heart. Poly-ε-caprolactone (PCL) scaffolds can be utilized to deliver viral vectors to the cartilage, bone, as well as to the brain [398]. Alginate and gelatin can deliver viral as well as non-viral vectors for the treatment of renal disorders, diabetes, tumors, ischemia, and can be utilized to target cartilage and muscles. Fibrin glue can deliver vectors targeting tissues such as bone, cartilage, blood vessels, and skin. Collagen as well as silk elastin like protein can deliver viral as well as non-viral vectors in muscles, blood vessels, bone, skin, and the brain for the therapy of tumors and ischemia. Hydrogels consisting of pluronic can deliver viral and non-viral vectors targeting cartilage and brain for the treatment of tumors. Self-assembling peptide hydrogels can deliver viral vectors targeting cartilage. Oligo (poly (ethylene glycol) fumarate) (OPF) hydrogels are being studied for controlled release of non-viral vectors. Poly(lactic-glycolic) acid (PLGA)-based gels can deliver viral as well as non-viral vectors for the treatment of tumors and wounds and can target blood vessels and the skin [398].
Selection of vectors is important and AAV vectors are less immunogenic compared to other viral vectors. However, the nucleic acid sequence and the capsid proteins delivered are capable of triggering our immune system by secreting neutralizing or non-neutralizing antibodies which in turn either eliminates transduced cells or opsonize the viral particles thereby diminishing the clinical efficacy of AAV-mediated therapeutic agents. There comes a challenge of how to deliver therapeutic dose of AAV in patients with an immunological memory against AAV. This can be overcome by the selection of specific AAV variants that are not yet circulated in human population so that there can be lesser chances of developing memory responses against T cells and neutralizing antibodies. Another option to minimize immunological responses can be the selection of specific administration routes for different therapeutic strategies [54].
Advancements in DNA sequencing have given the scientific community novel insights on how to deal with numerous diseases including genetic mutated neurological diseases. Primary bacterial research has paved way for development of CRISPR/Cas9 technology which gives scientific community possibilities of gene editing from any region of genome. An amalgam of personalized and precision medicine can help the physicians in choosing targeted therapy based on investigation of pathological problems prior to clinical symptom appearance. Next-generation sequencing (NGS) technology is another tool that could ease the diagnostic procedure through its accuracy, increased data reliability, and speed. Currently there are challenges such as difficulty in the management of large quantity of information and interpretation of the results, time requirement for sample preparation, and also necessity for developing a user-friendly software for analysis.
Concluding Remarks and Future Perspectives
There is unequivocal evidence that gene therapy has created new possibilities for treating many neurological disorders. These advances have been particularly prominent for treating AD, PD, ALS, SMA, spinocerebellar ataxia, epilepsy, HD, and stroke, wherein limited success is observed with conventional therapies. However, all delivery systems selected have pros and cons, and therefore, the assessment by clinical trials with therapeutic efficiency remains elusive. Among the various vectors investigated, AAV9 is economical with efficient transduction patterns and high neurotropic effects. The concept of hybrid vectors is that viral vectors are engineered genetically containing quality of more than one vector and can be beneficial in maintaining the stability of therapeutic DNA, and transgene within the cell. Thus, they add up advantages of avoiding repeated administration of drugs, and defective gene products can be produced stably. Vexosomes make use of this concept and are a novel system of gene delivery, whereby exosomes and the viral vector, for instance, are the most commonly used AAV, both combining delivering both features for an enhanced targeted delivery of peptides or the gene of choice to the desired tissue. This system suggests significant advantages such as rational dose rate to the targeted tissue avoiding viral vector-associated toxicity and reduced incidence of injections.
Indeed, an efficacious therapeutic gene can provide improvements in delivery methods and new vectors for gene therapy. Identifying and examining new therapeutic genes to ensure a clear understanding of disease inception and development is an area of remarkable interest. Biomarkers’ identification is beneficial for early diagnosis and early intervention and will allow a proper therapeutic intervention and disease monitoring. Also, therapeutic approaches concentrating addition or replacement of genes to target neurodegenerative diseases are evolving rapidly along with the advancements in the knowledge of molecular pathophysiology of these diseases. The development of iMRI-CED made possible the precise delivery of therapy using vectors and allows real-time modulation. The neurosurgical field is utilizing these strategies translated from research to the market for clinically effective patient care. Acute and chronic CNS disorders with various pathologies need a combinatorial synergistic therapeutic approach to modulate the different pathological processes resulting in neurodegeneration. Cell therapy and ex vivo gene therapy together utilizing stem cells seem promising to deliver neuroprotective agents to the stressed brain. The therapeutic agents diffusing outside engraftment areas may result in extensive neuroprotection in several neurodegenerative disorders. The in-depth knowledge of disease processes and recent advancements in iPSC capable of autologous transplantation and progress in gene editing strategies can provide useful and safe gene therapy for neurodegenerative disorders in the future, which seems promising for treating such affections.
Acknowledgments
Not applicable.
Author Contribution
All authors have read and agreed to the published version of the manuscript.
Data Availability
All data and material are now available.
Declarations
This manuscript is according to the ethical standards.
Consent to participate
Not applicable.
Consent for Publication
All authors agreed with the submitted version of the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Della Grace Thomas Parambi, Email: dellajesto@ju.edu.sa.
Khalid Saad Alharbi, Email: kssalharbi@ju.edu.sa.
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com.
Natália Cruz-Martins, Email: ncmartins@med.up.pt.
Omnia Magdy, Email: omnia_mmh@yahoo.com.
Arafa Musa, Email: akmusa@ju.edu.sa.
Dibya Sundar Panda, Email: dspanda@ju.edu.sa.
Bijo Mathew, Email: bijomathew@aims.amrita.edu.
References
- 1.Weinberg MS, Samulski RJ, McCown TJ. Adeno-associated virus (AAV) gene therapy for neurological disease. Neuropharmacology. 2013;69:82–88. doi: 10.1016/j.neuropharm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Connor DM, Boulis NM. Gene therapy for neurodegenerative diseases. Trends Mol Med. 2015;21:504–512. doi: 10.1016/j.molmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Martier R, Konstantinova P. Gene Therapy for neurodegenerative diseases: slowing down the ticking clock. Front Neurosci. 2020;14:1002. doi: 10.3389/fnins.2020.580179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harilal S, Jose J, Kumar R, Unnikrishnan MK, Uddin MS, Mathew GE, Pratap R, Marathakam A, et al. Revisiting the Blood-brain barrier: a hard nut to crack in the transportation of drug molecules. Brain Res Bull. 2020;160:121–140. doi: 10.1016/j.brainresbull.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Holt CE, Martin KC, Schuman EM. Local translation in neurons: visualization and function. Nat Struct Mol Biol. 2019;26:557–566. doi: 10.1038/s41594-019-0263-5. [DOI] [PubMed] [Google Scholar]
- 6.Richardson RM, Varenika V, Forsayeth JR, Bankiewicz KS. Future applications: gene therapy. Neurosurg Clin N Am. 2009;20:205–210. doi: 10.1016/j.nec.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Horn JD, Pelphrey KA. Neuroimaging of the developing brain. Brain Imaging Behav. 2015;9:1–4. doi: 10.1007/s11682-015-9365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deverman BE, Ravina BM, Bankiewicz KS, Paul SM, Sah DW. Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov. 2018;17:641–659. doi: 10.1038/nrd.2018.110. [DOI] [PubMed] [Google Scholar]
- 9.Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, Gray SJ, Lowenstein PR, et al. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9:277–291. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene Therapy clinical trials worldwide to 2017: an update. J. Gene Med. 2018;20:e3015. doi: 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- 11.Sudhakar V, Richardson RM. Gene therapy for neurodegenerative diseases. Neurother J Am Soc Exp Neurother. 2019;16:166–175. doi: 10.1007/s13311-018-00694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidorova YA, Saarma M. Can growth factors cure Parkinson’s disease? Trends Pharmacol. Sci. 2020;41(12):909–922. doi: 10.1016/j.tips.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Akhtar AA, Gowing G, Kobritz N, Savinoff SE, Garcia L, Saxon D, Cho N, Kim G, et al. Inducible expression of GDNF in transplanted IPSC-derived neural progenitor cells. Stem Cell Rep. 2018;10:1696–1704. doi: 10.1016/j.stemcr.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gantner CW, de Luzy IR, Kauhausen JA, Moriarty N, Niclis JC, Bye CR, Penna V, Hunt CP, et al. Viral delivery of GDNF promotes functional integration of human stem cell grafts in Parkinson’s disease. Cell Stem Cell. 2020;26:511–526. doi: 10.1016/j.stem.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Gowing G, Svendsen S, Svendsen CN. Ex vivo gene therapy for the treatment of neurological disorders. Prog Brain Res. 2017;230:99–132. doi: 10.1016/bs.pbr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Savić N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016;168:15–21. doi: 10.1016/j.trsl.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Behrstock S, Ebert A, McHugh J, Vosberg S, Moore J, Schneider B, Capowski E, Hei D, et al. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther. 2006;13:379–388. doi: 10.1038/sj.gt.3302679. [DOI] [PubMed] [Google Scholar]
- 18.Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN. GDNF Delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 19.Naldini L. Ex Vivo Gene Transfer and correction for cell-based therapies. Nat Rev Genet. 2011;12:301–315. doi: 10.1038/nrg2985. [DOI] [PubMed] [Google Scholar]
- 20.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Wang D-A. Viral vector-mediated transgenic cell therapy in regenerative medicine: safety of the process. Expert Opin Biol Ther. 2015;15:559–567. doi: 10.1517/14712598.2015.995086. [DOI] [PubMed] [Google Scholar]
- 22.Barrett R, Ornelas L, Yeager N, Mandefro B, Sahabian A, Lenaeus L, Targan SR, Svendsen CN, et al. Reliable generation of induced pluripotent stem cells from human lymphoblastoid cell lines. Stem Cells Transl Med. 2014;3:1429–1434. doi: 10.5966/sctm.2014-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaja MD, Rosenberg MB, Yoshida K, Gage FH. Somatic gene transfer of nerve growth factor promotes the survival of axotomized septal neurons and the regeneration of their axons in adult rats. J Neurosci. 1992;12:2849–2864. doi: 10.1523/JNEUROSCI.12-07-02849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuszynski M, Roberts J, Senut M, U HS, Gage F. Gene therapy in the adult primate brain: intraparenchymal grafts of cells genetically modified to produce nerve growth factor prevent cholinergic neuronal degeneration. Gene Ther. 1996;3(4):305–314. [PubMed] [Google Scholar]
- 26.Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, et al. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp Hematol. 2008;36:1035–1046. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Amemori T, Jendelova P, Ruzicka J, Urdzikova LM, Sykova E. Alzheimer’s disease: mechanism and approach to cell therapy. Int J Mol Sci. 2015;16:26417–26451. doi: 10.3390/ijms161125961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanna T, Sachan V. Mesenchymal stem cells: potential in treatment of neurodegenerative diseases. Curr Stem Cell Res Ther. 2014;9:513–521. doi: 10.2174/1574888X09666140923101110. [DOI] [PubMed] [Google Scholar]
- 29.Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/S0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 30.Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26:125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 31.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:l1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 32.de Miguel-Beriain I. The Ethics of Stem Cells Revisited. Adv Drug Deliv Rev. 2015;82:176–180. doi: 10.1016/j.addr.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, Hayashi T, Onoe H, et al. Direct Comparison of autologous and allogeneic transplantation of IPSC-derived neural cells in the brain of a nonhuman primate. Stem Cell Rep. 2013;1:283–292. doi: 10.1016/j.stemcr.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svendsen CN. Back to the future: how human induced pluripotent stem cells will transform regenerative medicine. Hum Mol Genet. 2013;22:R32–R38. doi: 10.1093/hmg/ddt379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirzaei H, Sahebkar A, Jaafari MR, Hadjati J, Javanmard SH, Mirzaei HR, Salehi R. PiggyBac as a novel vector in cancer gene therapy: current perspective. Cancer Gene Ther. 2016;23:45–47. doi: 10.1038/cgt.2015.68. [DOI] [PubMed] [Google Scholar]
- 36.Woodard LE, Wilson MH. PiggyBac-Ing models and new therapeutic strategies. Trends Biotechnol. 2015;33:525–533. doi: 10.1016/j.tibtech.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eid A, Mahfouz MM. Genome editing: the road of CRISPR/Cas9 from bench to clinic. Exp Mol Med. 2016;48:e265–e265. doi: 10.1038/emm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park C-Y, Lee DR, Sung JJ, Kim D-W. Genome-Editing technologies for gene correction of hemophilia. Hum Genet. 2016;135:977–981. doi: 10.1007/s00439-016-1699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai SQ, Joung JK. Defining and improving the genome-wide specificities of CRISPR–Cas9 nucleases. Nat Rev Genet. 2016;17:300–312. doi: 10.1038/nrg.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Im W, Moon J, Kim M. Applications of CRISPR/Cas9 for gene editing in hereditary movement disorders. J Mov Disord. 2016;9:136. doi: 10.14802/jmd.16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kay MA, Liu D, Hoogerbrugge PM. Gene therapy. Proc. Natl. Acad. Sci. 1997;94:12744LP–12746. doi: 10.1073/pnas.94.24.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingusci S, Verlengia G, Soukupova M, Zucchini S, Simonato M. Gene Therapy tools for brain diseases. Front Pharmacol. 2019;10:724. doi: 10.3389/fphar.2019.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shillitoe EJ. Gene Therapy: The End of the Rainbow? Head Neck Oncol. 2009;1:7. doi: 10.1186/1758-3284-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teschemacher AG, Wang S, Lonergan T, Duale H, Waki H, Paton JFR, Kasparov S. Targeting specific neuronal populations using adeno- and lentiviral vectors: applications for imaging and studies of cell function. Exp Physiol. 2005;90:61–69. doi: 10.1113/expphysiol.2004.028191. [DOI] [PubMed] [Google Scholar]
- 45.Bourdenx M, Dutheil N, Bezard E, Dehay B. Systemic gene delivery to the central nervous system using adeno-associated virus. Front Mol Neurosci. 2014;7:50. doi: 10.3389/fnmol.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Artusi S, Miyagawa Y, Goins WF, Cohen JB, Glorioso JC. Herpes Simplex virus vectors for gene transfer to the central nervous system. Dis Basel Switz. 2018;6:74. doi: 10.3390/diseases6030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, Zabner J, Ghodsi A, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kremer EJ, Perricaudet M. Adenovirus and Adeno-associated virus mediated gene transfer. Br Med Bull. 1995;51:31–44. doi: 10.1093/oxfordjournals.bmb.a072951. [DOI] [PubMed] [Google Scholar]
- 49.Slack RS, Miller FD. Viral vectors for modulating gene expression in neurons. Curr Opin Neurobiol. 1996;6:576–583. doi: 10.1016/S0959-4388(96)80088-2. [DOI] [PubMed] [Google Scholar]
- 50.Frampton AR, Goins WF, Nakano K, Burton EA, Glorioso JC. HSV Trafficking and development of gene therapy vectors with applications in the nervous system. Gene Ther. 2005;12:891–901. doi: 10.1038/sj.gt.3302545. [DOI] [PubMed] [Google Scholar]
- 51.Jain KK. Applications of Biotechnology in Neurology. Berlin: Springer; 2013. Gene therapy of neurological disorders; pp. 383–476. [Google Scholar]
- 52.Wang D, Tai PW, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortolano S, Spuch C, Navarro C. Present and future of adeno associated virus based gene therapy approaches. Recent Pat Endocr Metab Immune Drug Discov. 2012;6:47–66. doi: 10.2174/187221412799015245. [DOI] [PubMed] [Google Scholar]
- 54.Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dayton RD, Wang DB, Klein RL. The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert Opin Biol Ther. 2012;12:757–766. doi: 10.1517/14712598.2012.681463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haery L, Deverman BE, Matho KS, Cetin A, Woodard K, Cepko C, Guerin KI, Rego MA, et al. Adeno-associated virus technologies and methods for targeted neuronal manipulation. Front Neuroanat. 2019;13:93. doi: 10.3389/fnana.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tervo DGR, Hwang B-Y, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, et al. A Designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92:372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu G, Martins IH, Chiorini JA, Davidson BL. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Gene Ther. 2005;12:1503–1508. doi: 10.1038/sj.gt.3302554. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Wang R, Meng Q, Li G, Hu G, Dou W, Li Z, Zhang Z. Intra-ventricular infusion of RAAV1-EGFP Resulted in transduction in multiple regions of adult rat brain: a comparative study with RAAV2 and RAAV5 Vectors. Brain Res. 2006;1122:1–9. doi: 10.1016/j.brainres.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 60.Davidsson M, Wang G, Aldrin-Kirk P, Cardoso T, Nolbrant S, Hartnor M, Parmar M, Björklund T (2018) Barcoded rational AAV vector evolution enables systematic in vivo mapping of peptide binding motifs. bioRxiv 335372.
- 61.Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. 2020;21:255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 62.Davidsson M, Wang G, Aldrin-Kirk P, Cardoso T, Nolbrant S, Hartnor M, Mudannayake J, Parmar M, et al. A systematic capsid evolution approach performed in vivo for the design of AAV vectors with tailored properties and tropism. Proc Natl Acad Sci. 2019;116:27053–27062. doi: 10.1073/pnas.1910061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pillay S, Zou W, Cheng F, Puschnik AS, Meyer NL, Ganaie SS, Deng X, Wosen JE, et al. Adeno-associated virus (AAV) serotypes have distinctive interactions with domains of the cellular AAV receptor. J Virol. 2017;91:e00391–17. doi: 10.1128/JVI.00391-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, Beyer J, Forsayeth J, et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. 2013;20:348–352. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, Aminoff MJ. Results from a Phase I Safety Trial of HAADC Gene Therapy for Parkinson Disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 67.Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2–neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 68.Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, VanBrocklin HF, Wright JF, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K, Kawakami T, Urabe M, et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther J Am Soc Gene Ther. 2010;18:1731–1735. doi: 10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rafii MS, Tuszynski MH, Thomas RG, Barba D, Brewer JB, Rissman RA, Siffert J, Aisen PS, et al. Adeno-associated viral vector (serotype 2)-nerve growth factor for patients with Alzheimer disease: a randomized clinical trial. JAMA Neurol. 2018;75:834–841. doi: 10.1001/jamaneurol.2018.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 72.LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, et al. AAV2-GAD Gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/s1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 73.Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J, Bankiewicz KS. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc Natl Acad Sci. 2009;106:2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dreyer J-L. Lentiviral vector-mediated gene transfer and rna silencing technology in neuronal dysfunctions. Lentivirus Gene Eng Protoc. 2010;47:3–35. doi: 10.1007/978-1-60761-533-0_1. [DOI] [PubMed] [Google Scholar]
- 75.Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. The Lancet. 2014;383:1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 76.Gray SJ, Woodard KT, Samulski RJ. Viral vectors and delivery strategies for CNS gene therapy. Ther Deliv. 2010;1:517–534. doi: 10.4155/tde.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaiserman A, De Falco E, Koliada A, Maslova O, Balistreri CR. Anti-ageing gene therapy: not so far away? Ageing Res Rev. 2019;56:100977. doi: 10.1016/j.arr.2019.100977. [DOI] [PubMed] [Google Scholar]
- 78.Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35–47. doi: 10.1016/S0163-7258(98)00020-5. [DOI] [PubMed] [Google Scholar]
- 79.Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003;21:117–122. doi: 10.1016/S0167-7799(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 80.Lundstrom K. Viral Vectors in Gene Therapy. Diseases. 2018;6:42. doi: 10.3390/diseases6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamimura K, Suda T, Zhang G, Liu D. Advances in gene delivery systems. Pharm Med. 2011;25:293–306. doi: 10.1007/BF03256872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen W, Hu Y, Ju D. Gene therapy for neurodegenerative disorders: advances, insights and prospects. Acta Pharm Sin B. 2020;10:1347–1359. doi: 10.1016/j.apsb.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramamoorth M, Narvekar A. Non viral vectors in gene therapy-an overview. J. Clin. Diagn. Res. 2015;9:GE01. doi: 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11:671–681. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 86.Jinturkar KA, Rathi MN, Misra A. Challenges in delivery of therapeutic genomics and proteomics. Amsterdam: Elsevier; 2011. Gene delivery using physical methods; pp. 83–126. [Google Scholar]
- 87.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-Enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varenika V, Dickinson P, Bringas J, LeCouteur R, Higgins R, Park J, Fiandaca M, Berger M, et al. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. J Neurosurg. 2008;109:874–880. doi: 10.3171/JNS/2008/109/11/0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varenika V, Kells AP, Valles F, Hadaczek P, Forsayeth J, Bankiewicz KS. Controlled dissemination of aav vectors in the primate brain. Prog Brain Res. 2009;175:163–172. doi: 10.1016/S0079-6123(09)17511-8. [DOI] [PubMed] [Google Scholar]
- 90.Valles F, Fiandaca MS, Bringas J, Dickinson P, LeCouteur R, Higgins R, Berger M, Forsayeth J, et al. Anatomic compression caused by high-volume convection-enhanced delivery to the brain. Neurosurgery. 2009;65:579–586. doi: 10.1227/01.NEU.0000350229.77462.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valles F, Fiandaca MS, Eberling JL, Starr PA, Larson PS, Christine CW, Forsayeth J, Richardson RM, Su X, Aminoff MJ. Qualitative Imaging of adeno-associated virus serotype 2–human aromatic L-amino acid decarboxylase gene therapy in a phase I study for the treatment of Parkinson disease. Neurosurgery. 2010;67:1377–1385. doi: 10.1227/NEU.0b013e3181f53a5c. [DOI] [PubMed] [Google Scholar]
- 92.Yin D, Richardson RM, Fiandaca MS, Bringas J, Forsayeth J, Berger MS, Bankiewicz KS. Cannula placement for effective convection-enhanced delivery in the nonhuman primate thalamus and brainstem: implications for clinical delivery of therapeutics. J Neurosurg. 2010;113:240–248. doi: 10.3171/2010.2.JNS091744. [DOI] [PubMed] [Google Scholar]
- 93.Yin D, Valles FE, Fiandaca MS, Bringas J, Gimenez F, Berger MS, Forsayeth J, Bankiewicz KS. Optimal region of the putamen for image-guided convection-enhanced delivery of therapeutics in human and non-human primates. Neuroimage. 2011;54:S196–S203. doi: 10.1016/j.neuroimage.2009.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richardson RM, Kells AP, Martin AJ, Larson PS, Starr PA, Piferi PG, Bates G, Tansey L, Rosenbluth KH, Bringas JR. Novel platform for mri-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotact Funct Neurosurg. 2011;89:141–151. doi: 10.1159/000323544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su X, Kells AP, Salegio EA, Richardson RM, Hadaczek P, Beyer J, Bringas J, Pivirotto P, Forsayeth J, Bankiewicz KS. Real-time MR imaging with gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Mol Ther. 2010;18:1490–1495. doi: 10.1038/mt.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richardson RM, Kells AP, Rosenbluth KH, Salegio EA, Fiandaca MS, Larson PS, Starr PA, Martin AJ, Lonser RR, Federoff HJ. Interventional MRI-guided putaminal delivery of AAV2-GDNF for a Planned clinical trial in Parkinson’s disease. Mol Ther. 2011;19:1048–1057. doi: 10.1038/mt.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jagannath A, Wood M. RNA interference based gene therapy for neurological disease. Brief Funct Genomic Proteomic. 2007;6:40–49. doi: 10.1093/bfgp/elm005. [DOI] [PubMed] [Google Scholar]
- 98.Nyamay’Antu A, Dumont M, Kedinger V, Erbacher P. Non-viral vector mediated gene delivery: the outsider to watch out for in gene therapy. Cell Gene Ther Insights. 2019;5:51–57. doi: 10.18609/cgti.2019.007. [DOI] [Google Scholar]
- 99.Hart SL. Multifunctional nanocomplexes for gene transfer and gene therapy. Cell Biol Toxicol. 2010;26:69–81. doi: 10.1007/s10565-009-9141-y. [DOI] [PubMed] [Google Scholar]
- 100.Vivien É, Oudrhiri N, Vigneron J-P, Hauchecorne M, Ramasawmy R, Riquier S, Toury R, Fabrega S, Navarro J, Lehn J-M, et al. Vecteurs Synthétiques Pour Le Transfert de Gènes: État Actuel et Perspectives. Ann Inst Pasteur Actual. 1999;10:301–312. doi: 10.1016/S0924-4204(00)80002-5. [DOI] [Google Scholar]
- 101.Kremer EJ, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J Virol. 2000;74:505–512. doi: 10.1128/jvi.74.1.505-512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramamoorth M, Narvekar A. Non viral vectors in gene therapy- an overview. J Clin Diagn Res. 2015;9:1–6. doi: 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pinto-González Howell D, Krieser RJ, Eastman A, Barry MA. Deoxyribonuclease II is a lysosomal barrier to transfection. Mol Ther. 2003;8:957–963. doi: 10.1016/j.ymthe.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 104.Matsumoto Y, Itaka K, Yamasoba T, Kataoka K. Intranuclear fluorescence resonance energy transfer analysis of plasmid DNA decondensation from nonviral gene carriers. J Gene Med. 2009;11:615–623. doi: 10.1002/jgm.1338. [DOI] [PubMed] [Google Scholar]
- 105.Li S-D, Huang L. Gene therapy progress and prospects: non-viral gene therapy by systemic delivery. Gene Ther. 2006;13:1313–1319. doi: 10.1038/sj.gt.3302838. [DOI] [PubMed] [Google Scholar]
- 106.Dufès C, Uchegbu IF, Schätzlein AG. Dendrimers in gene delivery. Adv Drug Deliv Rev. 2005;57:2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 107.Huang R-Q, Qu Y-H, Ke W-L, Zhu J-H, Pei Y-Y, Jiang C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J. 2007;21:1117–1125. doi: 10.1096/fj.06-7380com. [DOI] [PubMed] [Google Scholar]
- 108.Knorr V, Allmendinger L, Walker GF, Paintner FF, Wagner E. An acetal-based PEGylation reagent for PH-sensitive shielding of DNA polyplexes. Bioconjug Chem. 2007;18:1218–1225. doi: 10.1021/bc060327a. [DOI] [PubMed] [Google Scholar]
- 109.Wolff JA, Rozema DB. Breaking the bonds: non-viral vectors become chemically dynamic. Mol Ther. 2008;16:8–15. doi: 10.1038/sj.mt.6300326. [DOI] [PubMed] [Google Scholar]
- 110.Piest M, Lin C, Mateos-Timoneda MA, Lok MC, Hennink WE, Feijen J, Engbersen JFJ. Novel Poly(Amido Amine)s with Bioreducible Disulfide Linkages in Their Diamino-Units: Structure Effects and in Vitro Gene Transfer Properties. J Controlled Release. 2008;130:38–45. doi: 10.1016/j.jconrel.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 111.Slonczewski JL, Foster JW, Foster E. Microbiology: An Evolving Science Fifth International Student Edition with Ebook, Smartwork5, Animations ETopics and EAppendices. New York: WW Norton & Company; 2020. [Google Scholar]
- 112.Voet D, Voet JG, Pratt CW. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken: John Wiley & Sons; 2016. [Google Scholar]
- 113.Zheng C, Baum BJ. Gene Therapy Protocols. Springer; 2008. Evaluation of promoters for use in tissue-specific gene delivery; pp. 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skinner GM, Baumann CG, Quinn DM, Molloy JE, Hoggett JG. Promoter Binding, initiation, and elongation by bacteriophage T7 RNA polymerase: a single-molecule view of the transcription cycle. J Biol Chem. 2004;279:3239–3244. doi: 10.1074/jbc.M310471200. [DOI] [PubMed] [Google Scholar]
- 115.Wyman TC, Rohrer DC, Kirigiti P, Nichols HV, Pilcher KY, Nilaver G, Machida CA. Promoter-activated expression of nerve growth factor for treatment of neurodegenerative diseases. Gene Ther. 1999;6:1648–1660. doi: 10.1038/sj.gt.3300989. [DOI] [PubMed] [Google Scholar]
- 116.Da Silva NA, Srikrishnan S. Introduction and expression of genes for metabolic engineering applications in Saccharomyces cerevisiae. FEMS Yeast Res. 2012;12:197–214. doi: 10.1111/j.1567-1364.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 117.Pfeiffer BD, Ngo T-TB, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewis LK, Jenkins ME, Mount DW. Isolation of DNA damage-inducible promoters in Escherichia coli: regulation of PolB (DinA), DinG, and DinH by LexA Repressor. J Bacteriol. 1992;174:3377–3385. doi: 10.1128/jb.174.10.3377-3385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Borghi L. Plant Developmental Biology. Berlin: Springer; 2010. Inducible gene expression systems for plants; pp. 65–75. [DOI] [PubMed] [Google Scholar]
- 120.Schmidl SR, Sheth RU, Wu A, Tabor JJ. Refactoring and optimization of light-switchable Escherichia coli two-component systems. ACS Synth Biol. 2014;3:820–831. doi: 10.1021/sb500273n. [DOI] [PubMed] [Google Scholar]
- 121.Tabor JJ, Levskaya A, Voigt CA. Multichromatic control of gene expression in Escherichia coli. J Mol Biol. 2011;405:315–324. doi: 10.1016/j.jmb.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toscano MG, Romero Z, Munoz P, Cobo M, Benabdellah K, Martin F. Physiological and tissue-specific vectors for treatment of inherited diseases. Gene Ther. 2011;18:117–127. doi: 10.1038/gt.2010.138. [DOI] [PubMed] [Google Scholar]
- 123.Venter M. Synthetic promoters: genetic control through Cis engineering. Trends Plant Sci. 2007;12:118–124. doi: 10.1016/j.tplants.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 124.Kluge J, Terfehr D, Kück U. Inducible promoters and functional genomic approaches for the genetic engineering of filamentous fungi. Appl Microbiol Biotechnol. 2018;102:6357–6372. doi: 10.1007/s00253-018-9115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP Promoter Directs astrocyte-specific expression in transgenic mice. J Neurosci Off J Soc Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karpati G, Lochmüller H, Nalbantoglu J, Durham H. The principles of gene therapy for the nervous system. Trends Neurosci. 1996;19:49–54. doi: 10.1016/0166-2236(96)89620-2. [DOI] [PubMed] [Google Scholar]
- 127.Salbaum JM, Weidemann A, Lemaire HG, Masters CL, Beyreuther K. The promoter of Alzheimer’s disease amyloid A4 precursor gene. EMBO J. 1988;7:2807–2813. doi: 10.1002/j.1460-2075.1988.tb03136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohyagi Y, Asahara H, Chui D-H, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H. Intracellular Aβ42 activates P53 promoter: a pathway to neurodegeneration in Alzheimer’s disease. FASEB J. 2005;19:1–29. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- 129.Bizzarro A, Seripa D, Acciarri A, Matera MG, Pilotto A, Tiziano FD, Brahe C, Masullo C. The complex interaction between APOE promoter and AD: an Italian case–control study. Eur J Hum Genet. 2009;17:938–945. doi: 10.1038/ejhg.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tycko B, Lee JH, Ciappa A, Saxena A, Li C-M, Feng L, Arriaga A, Stern Y, Lantigua R, Shachter N. APOE and APOC1 promoter polymorphisms and the risk of Alzheimer disease in African American and Caribbean Hispanic Individuals. Arch Neurol. 2004;61:1434–1439. doi: 10.1001/archneur.61.9.1434. [DOI] [PubMed] [Google Scholar]
- 131.Segat L, Pontillo A, Annoni G, Trabattoni D, Vergani C, Clerici M, Arosio B, Crovella S. PIN1 Promoter polymorphisms are associated with Alzheimer’s disease. Neurobiol Aging. 2007;28:69–74. doi: 10.1016/j.neurobiolaging.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 132.Ji W, Zhang Y. The association of MPO gene promoter polymorphisms with Alzheimer’s disease risk in Chinese Han Population. Oncotarget. 2017;8:107870. doi: 10.18632/oncotarget.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wettergren EE, Gussing F, Quintino L, Lundberg C. Novel disease-specific promoters for use in gene therapy for Parkinson’s disease. Neurosci Lett. 2012;530:29–34. doi: 10.1016/j.neulet.2012.09.059. [DOI] [PubMed] [Google Scholar]
- 134.Ay M, Jin H, Harischandra DS, Asaithambi A, Kanthasamy A, Anantharam V, Kanthasamy AG. Molecular cloning, epigenetic regulation, and functional characterization of Prkd1 gene promoter in dopaminergic cell culture models of Parkinson’s disease. J Neurochem. 2015;135:402–415. doi: 10.1111/jnc.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Suwelack D, Hurtado-Lorenzo A, Millan E, Gonzalez-Nicolini V, Wawrowsky K, Lowenstein PR, Castro MG. Neuronal Expression of the Transcription Factor Gli1 Using the Tα1 α-Tubulin Promoter Is Neuroprotective in an Experimental Model of Parkinson’s Disease. Gene Ther. 2004;11:1742–1752. doi: 10.1038/sj.gt.3302377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harlan BA, Pehar M, Killoy KM, Vargas MR. Enhanced SIRT6 Activity Abrogates the Neurotoxic Phenotype of Astrocytes Expressing ALS-Linked Mutant SOD1. FASEB J. 2019;33:7084–7091. doi: 10.1096/fj.201802752R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Finiels F, y Ribotta MG, Barkats M, Samolyk M-L, Robert JJ, Privat A, Revah F, Mallet J. Specific and Efficient Genetransfer Strategy Offers New Potentialities for the Treatment of Motor Neurone Diseases. Neuroreport. 1995;6:2473–2478. doi: 10.1097/00001756-199512150-00009. [DOI] [PubMed] [Google Scholar]
- 138.Ghadge G, Roos R, Kang U, Wollmann R, Fishman P, Kalynych A, Barr E, Leiden J. CNS Gene Delivery by Retrograde Transport of Recombinant Replication-Defective Adenoviruses. Gene Ther. 1995;2(2):132–137. [PubMed] [Google Scholar]
- 139.Mingozzi F, High KA. Immune Responses to AAV Vectors: Overcoming Barriers to Successful Gene Therapy. Blood J Am Soc Hematol. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. AAV Serotype 2 Vectors Preferentially Integrate into Active Genes in Mice. Nat Genet. 2003;34:297–302. doi: 10.1038/ng1179. [DOI] [PubMed] [Google Scholar]
- 141.Chandler RJ, Sands MS, Venditti CP. Recombinant Adeno-Associated Viral Integration and Genotoxicity: Insights from Animal Models. Hum Gene Ther. 2017;28:314–322. doi: 10.1089/hum.2017.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nault J-C, Datta S, Imbeaud S, Franconi A, Mallet M, Couchy G, Letouzé E, Pilati C, Verret B, Blanc J-F. Recurrent AAV2-Related Insertional Mutagenesis in Human Hepatocellular Carcinomas. Nat Genet. 2015;47:1187–1193. doi: 10.1038/ng.3389. [DOI] [PubMed] [Google Scholar]
- 143.Scott LJ. Alipogene Tiparvovec: A Review of Its Use in Adults with Familial Lipoprotein Lipase Deficiency. Drugs. 2015;75:175–182. doi: 10.1007/s40265-014-0339-9. [DOI] [PubMed] [Google Scholar]
- 144.Salmon F, Grosios K, Petry H. Safety Profile of Recombinant Adeno-Associated Viral Vectors: Focus on Alipogene Tiparvovec (Glybera®) Expert Rev Clin Pharmacol. 2014;7:53–65. doi: 10.1586/17512433.2014.852065. [DOI] [PubMed] [Google Scholar]
- 145.Wang D, Zhong L, Nahid MA, Gao G. The Potential of Adeno-Associated Viral Vectors for Gene Delivery to Muscle Tissue. Expert Opin Drug Deliv. 2014;11:345–364. doi: 10.1517/17425247.2014.871258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hocquemiller M, Giersch L, Audrain M, Parker S, Cartier N. Adeno-Associated Virus-Based Gene Therapy for CNS Diseases. Hum Gene Ther. 2016;27:478–496. doi: 10.1089/hum.2016.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ojala DS, Amara DP, Schaffer DV. Adeno-Associated Virus Vectors and Neurological Gene Therapy. Neuroscientist. 2015;21:84–98. doi: 10.1177/1073858414521870. [DOI] [PubMed] [Google Scholar]
- 148.Yang B, Li S, Wang H, Guo Y, Gessler DJ, Cao C, Su Q, Kramer J, Zhong L, Ahmed SS. Global CNS Transduction of Adult Mice by Intravenously Delivered RAAVrh. 8 and RAAVrh. 10 and Nonhuman Primates by RAAVrh. 10. Mol. Ther. 2014;22:1299–1309. doi: 10.1038/mt.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gray SJ, Kalburgi SN, McCown TJ, Samulski RJ. Global CNS Gene Delivery and Evasion of Anti-AAV-Neutralizing Antibodies by Intrathecal AAV Administration in Non-Human Primates. Gene Ther. 2013;20:450–459. doi: 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang H, Yang B, Qiu L, Yang C, Kramer J, Su Q, Guo Y, Brown RH, Jr, Gao G, Xu Z. Widespread Spinal Cord Transduction by Intrathecal Injection of RAAV Delivers Efficacious RNAi Therapy for Amyotrophic Lateral Sclerosis. Hum Mol Genet. 2014;23:668–681. doi: 10.1093/hmg/ddt454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saraiva J, Nobre RJ, de Almeida LP. Gene Therapy for the CNS Using AAVs: The Impact of Systemic Delivery by AAV9. J Controlled Release. 2016;241:94–109. doi: 10.1016/j.jconrel.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 152.Selkoe DJ, Hardy J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Anand R, Kaushal A, Wani WY, Gill KD. Road to Alzheimer’s Disease: The Pathomechanism Underlying. Pathobiology. 2012;79:55–71. doi: 10.1159/000332218. [DOI] [PubMed] [Google Scholar]
- 154.St-Amour I, Cicchetti F, Calon F. Immunotherapies in Alzheimer’s Disease: Too Much, Too Little, Too Late or off-Target? Acta Neuropathol (Berl) 2016;131:481–504. doi: 10.1007/s00401-015-1518-9. [DOI] [PubMed] [Google Scholar]
- 155.Shimada M, Abe S, Takahashi T, Shiozaki K, Okuda M, Mizukami H, Klinman DM, Ozawa K, Okuda K. Prophylaxis and Treatment of Alzheimer’s Disease by Delivery of an Adeno-Associated Virus Encoding a Monoclonal Antibody Targeting the Amyloid Beta Protein. PloS One. 2013;8:e57606. doi: 10.1371/journal.pone.0057606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fish KM. Advances in Gene Therapy for Heart Failure. Discov Med. 2015;19:285–291. [PubMed] [Google Scholar]
- 157.Greenberg B. Gene Therapy for Heart Failure. J Cardiol. 2015;66:195–200. doi: 10.1016/j.jjcc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 158.Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Patients with Cardiac Disease (CUPID 2): A Randomised, Multinational, Double-Blind, Placebo-Controlled, Phase 2b Trial. The Lancet. 2016;387:1178–1186. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 159.Greenberg B, Yaroshinsky A, Zsebo KM, Butler J, Felker GM, Voors AA, Rudy JJ, Wagner K, Hajjar RJ. Design of a Phase 2b Trial of Intracoronary Administration of AAV1/SERCA2a in Patients with Advanced Heart Failure: The CUPID 2 Trial (Calcium up-Regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease Phase 2b) JACC Heart Fail. 2014;2:84–92. doi: 10.1016/j.jchf.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 160.Griesenbach U, Pytel KM, Alton EW. Cystic Fibrosis Gene Therapy in the UK and Elsewhere. Hum Gene Ther. 2015;26:266–275. doi: 10.1089/hum.2015.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burney TJ, Davies JC. Gene Therapy for the Treatment of Cystic Fibrosis. Appl Clin Genet. 2012;5:29. doi: 10.2147/TACG.S8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schuster BS, Kim AJ, Kays JC, Kanzawa MM, Guggino WB, Boyle MP, Rowe SM, Muzyczka N, Suk JS, Hanes J. Overcoming the Cystic Fibrosis Sputum Barrier to Leading Adeno-Associated Virus Gene Therapy Vectors. Mol Ther. 2014;22:1484–1493. doi: 10.1038/mt.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Limberis MP, Adam VS, Wong G, Gren J, Kobasa D, Ross TM, Kobinger GP, Tretiakova A, Wilson JM. Intranasal Antibody Gene Transfer in Mice and Ferrets Elicits Broad Protection against Pandemic Influenza. Sci. Transl. Med. 2013;5:187ra72. doi: 10.1126/scitranslmed.3006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 Preferentially Targets Neonatal Neurons and Adult Astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hudry E, Vandenberghe LH. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron. 2019;101:839–862. doi: 10.1016/j.neuron.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 166.Lukashchuk V, Lewis KE, Coldicott I, Grierson AJ, Azzouz M. AAV9-Mediated Central Nervous System-Targeted Gene Delivery via Cisterna Magna Route in Mice. Mol Ther - Methods Clin Dev. 2016;3:15055. doi: 10.1038/mtm.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lykken EA, Shyng C, Edwards RJ, Rozenberg A, Gray SJ. Recent Progress and Considerations for AAV Gene Therapies Targeting the Central Nervous System. J Neurodev Disord. 2018;10:1–10. doi: 10.1186/s11689-018-9234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Palfi S, Gurruchaga JM, Lepetit H, Howard K, Ralph GS, Mason S, Gouello G, Domenech P, Buttery PC, Hantraye P. Long-Term Follow-up of a Phase I/II Study of ProSavin, a Lentiviral Vector Gene Therapy for Parkinson’s Disease. Hum Gene Ther Clin Dev. 2018;29:148–155. doi: 10.1089/humc.2018.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wurtman R. Biomarkers in the Diagnosis and Management of Alzheimer’s Disease. Metab - Clin Exp. 2015;64:S47–S50. doi: 10.1016/j.metabol.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 170.Bakkar N, Boehringer A, Bowser R. Use of Biomarkers in ALS Drug Development and Clinical Trials. Brain Res. 2015;1607:94–107. doi: 10.1016/j.brainres.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid Biomarkers in Alzheimer’s Disease. Trends Pharmacol Sci. 2015;36:297–309. doi: 10.1016/j.tips.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 172.Magdalinou N, Lees AJ, Zetterberg H. Cerebrospinal Fluid Biomarkers in Parkinsonian Conditions: An Update and Future Directions. J. Neurol. Neurosurg. Ampamp Psychiatry. 2014;85:1065–1075. doi: 10.1136/jnnp-2013-307539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee HJ, Lim IJ, Park SW, Kim YB, Ko Y, Kim SU. Human Neural Stem Cells Genetically Modified to Express Human Nerve Growth Factor (NGF) Gene Restore Cognition in the Mouse with Ibotenic Acid-Induced Cognitive Dysfunction. Cell Transplant. 2012;21:2487–2496. doi: 10.3727/096368912X638964. [DOI] [PubMed] [Google Scholar]
- 174.Wu S, Sasaki A, Yoshimoto R, Kawahara Y, Manabe T, Kataoka K, Asashima M, Yuge L. Neural Stem Cells Improve Learning and Memory in Rats with Alzheimer’s Disease. Pathobiology. 2008;75:186–194. doi: 10.1159/000124979. [DOI] [PubMed] [Google Scholar]
- 175.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller F-J, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural Stem Cells Improve Cognition via BDNF in a Transgenic Model of Alzheimer Disease. Proc Natl Acad Sci. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goldberg NR, Caesar J, Park A, Sedgh S, Finogenov G, Masliah E, Davis J, Blurton-Jones M. Neural Stem Cells Rescue Cognitive and Motor Dysfunction in a Transgenic Model of Dementia with Lewy Bodies through a BDNF-Dependent Mechanism. Stem Cell Rep. 2015;5:791–804. doi: 10.1016/j.stemcr.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stopa EG, Gonzalez A-M, Chorsky R, Corona RJ, Alvarez J, Bird ED, Baird A. Basic Fibroblast Growth Factor in Alzheimer’s Disease. Biochem Biophys Res Commun. 1990;171:690–696. doi: 10.1016/0006-291X(90)91201-3. [DOI] [PubMed] [Google Scholar]
- 178.Pascual-Lucas M, da Silva S, de Scala M, Garcia-Barroso C, González-Aseguinolaza G, Mulle C, Alberini CM, Cuadrado-Tejedor M, Garcia-Osta A. Insulin-like Growth Factor 2 Reverses Memory and Synaptic Deficits in APP Transgenic Mice. Embo Mol Med. 2014;6:1246–1262. doi: 10.15252/emmm.201404228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lipton JO, Sahin M. The Neurology of MTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zuleta A, Vidal RL, Armentano D, Parsons G, Hetz C. AAV-Mediated Delivery of the Transcription Factor XBP1s into the Striatum Reduces Mutant Huntingtin Aggregation in a Mouse Model of Huntington’s Disease. Biochem Biophys Res Commun. 2012;420:558–563. doi: 10.1016/j.bbrc.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 181.Du F, Yu Q, Yan S, Hu G, Lue L-F, Walker DG, Wu L, Yan SF, Tieu K, Yan SS. PINK1 Signalling Rescues Amyloid Pathology and Mitochondrial Dysfunction in Alzheimer’s Disease. Brain. 2017;140:3233–3251. doi: 10.1093/brain/awx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Blurton-Jones M, Spencer B, Michael S, Castello NA, Agazaryan AA, Davis JL, Müller F-J, Loring JF, Masliah E, LaFerla FM. Neural Stem Cells Genetically-Modified to Express Neprilysin Reduce Pathology in Alzheimer Transgenic Models. Stem Cell Res Ther. 2014;5:1–14. doi: 10.1186/scrt440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ghosh R, Tabrizi SJ. Gene Suppression Approaches to Neurodegeneration. Alzheimers Res Ther. 2017;9:1–13. doi: 10.1186/s13195-017-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hafez DM, Huang JY, Richardson JC, Masliah E, Peterson DA, Marr RA. F-Spondin Gene Transfer Improves Memory Performance and Reduces Amyloid-β Levels in Mice. Neuroscience. 2012;223:465–472. doi: 10.1016/j.neuroscience.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.López-Tobón A, Castro-Álvarez JF, Piedrahita D, Boudreau RL, Gallego-Gómez JC, Cardona-Gómez GP. Silencing of CDK5 as Potential Therapy for Alzheimer’s Disease. 2011. [DOI] [PubMed] [Google Scholar]
- 186.Cik M, Masure S, Lesage AS, Van der Linden I, Van Gompel P, Pangalos MN, Gordon RD, Leysen JE. Binding of GDNF and Neurturin to Human GDNF Family Receptor α 1 and 2: INFLUENCE OF CRET AND COOPERATIVE INTERACTIONS. J Biol Chem. 2000;275:27505–27512. doi: 10.1074/jbc.M000306200. [DOI] [PubMed] [Google Scholar]
- 187.Qu Y, Liu Y, Noor AF, Tran J, Li R. Characteristics and Advantages of Adeno-Associated Virus Vector-Mediated Gene Therapy for Neurodegenerative Diseases. Neural Regen Res. 2019;14:931. doi: 10.4103/1673-5374.250570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Emborg ME, Ebert AD, Moirano J, Peng S, Suzuki M, Capowski E, Joers V, Roitberg BZ, Aebischer P, Svendsen CN. GDNF-Secreting Human Neural Progenitor Cells Increase Tyrosine Hydroxylase and VMAT2 Expression in MPTP-Treated Cynomolgus Monkeys. Cell Transplant. 2008;17:383–395. doi: 10.3727/096368908784423300. [DOI] [PubMed] [Google Scholar]
- 189.Bockaert J, Marin P. MTOR in Brain Physiology and Pathologies. Physiol Rev. 2015;95:1157–1187. doi: 10.1152/physrev.00038.2014. [DOI] [PubMed] [Google Scholar]
- 190.Valdés P, Mercado G, Vidal RL, Molina C, Parsons G, Martinez A, Galleguillos D, Armentano D, Schneider BL, Hetz C. Control of Dopaminergic Neuron Survival by the Unfolded Protein Response Transcription Factor XBP1. Proc Natl Acad Sci. 2014;111:6804–6809. doi: 10.1073/pnas.1321845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and Endoplasmic Reticulum Stress Mediate Neurotoxicity of Pathological Prion Protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jin T, Gu Y, Zanusso G, Sy M, Kumar A, Cohen M, Gambetti P, Singh N. The Chaperone Protein BiP Binds to a Mutant Prion Protein and Mediates Its Degradation by the Proteasome. J Biol Chem. 2000;275:38699–38704. doi: 10.1074/jbc.M005543200. [DOI] [PubMed] [Google Scholar]
- 193.Manfredsson FP, Lewin AS, Mandel RJ. RNA Knockdown as a Potential Therapeutic Strategy in Parkinson’s Disease. Gene Ther. 2006;13:517–524. doi: 10.1038/sj.gt.3302669. [DOI] [PubMed] [Google Scholar]
- 194.Bongioanni P, Reali C, Sogos V (2004) Ciliary Neurotrophic Factor (CNTF) for Amyotrophic Lateral Sclerosis or Motor Neuron Disease. Cochrane Database Syst Rev [DOI] [PMC free article] [PubMed]
- 195.Pasquin S, Sharma M, Gauchat J-F. Ciliary Neurotrophic Factor (CNTF): New Facets of an Old Molecule for Treating Neurodegenerative and Metabolic Syndrome Pathologies. Cytokine Growth Factor Rev. 2015;26:507–515. doi: 10.1016/j.cytogfr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 196.Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF Secreting Human Neural Progenitor Cells Protect Dying Motor Neurons, but Not Their Projection to Muscle, in a Rat Model of Familial ALS. PloS One. 2007;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Krakora D, Mulcrone P, Meyer M, Lewis C, Bernau K, Gowing G, Zimprich C, Aebischer P, Svendsen CN, Suzuki M. Synergistic Effects of GDNF and VEGF on Lifespan and Disease Progression in a Familial ALS Rat Model. Mol Ther. 2013;21:1602–1610. doi: 10.1038/mt.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gravel C, Götz R, Lorrain A, Sendtner M. Adenoviral Gene Transfer of Ciliary Neurotrophic Factor and Brain-Derived Neurotrophic Factor Leads to Long-Term Survival of Axotomized Motor Neurons. Nat Med. 1997;3:765–770. doi: 10.1038/nm0797-765. [DOI] [PubMed] [Google Scholar]
- 199.Andrews J. Amyotrophic Lateral Sclerosis: Clinical Management and Research Update. Curr Neurol Neurosci Rep. 2009;9:59–68. doi: 10.1007/s11910-009-0010-0. [DOI] [PubMed] [Google Scholar]
- 200.Keifer OP, Jr, O’Connor DM, Boulis NM. Gene and Protein Therapies Utilizing VEGF for ALS. Pharmacol Ther. 2014;141:261–271. doi: 10.1016/j.pharmthera.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, Rao M, Eagle A, Matthews JC, Taksir TV. AAV4-Mediated Expression of IGF-1 and VEGF within Cellular Components of the Ventricular System Improves Survival Outcome in Familial ALS Mice. Mol Ther. 2010;18:2075–2084. doi: 10.1038/mt.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Passini MA, Bu J, Richards AM, Treleaven CM, Sullivan JA, O’Riordan CR, Scaria A, Kells AP, Samaranch L, San Sebastian W. Translational Fidelity of Intrathecal Delivery of Self-Complementary AAV9–Survival Motor Neuron 1 for Spinal Muscular Atrophy. Hum Gene Ther. 2014;25:619–630. doi: 10.1089/hum.2014.011. [DOI] [PubMed] [Google Scholar]
- 203.Meyer K, Ferraiuolo L, Schmelzer L, Braun L, McGovern V, Likhite S, Michels O, Govoni A, Fitzgerald J, Morales P, et al. Improving Single Injection CSF Delivery of AAV9-Mediated Gene Therapy for SMA: A Dose-Response Study in Mice and Nonhuman Primates. Mol Ther J Am Soc Gene Ther. 2015;23:477–487. doi: 10.1038/mt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AHM, et al. Rescue of the Spinal Muscular Atrophy Phenotype in a Mouse Model by Early Postnatal Delivery of SMN. Nat Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 205.Ashizawa T, Öz G, Paulson HL. Spinocerebellar Ataxias: Prospects and Challenges for Therapy Development. Nat Rev Neurol. 2018;14:590–605. doi: 10.1038/s41582-018-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Paradiso B, Marconi P, Zucchini S, Berto E, Binaschi A, Bozac A, Buzzi A, Mazzuferi M, Magri E, Mora GN, et al. Localized Delivery of Fibroblast Growth Factor–2 and Brain-Derived Neurotrophic Factor Reduces Spontaneous Seizures in an Epilepsy Model. Proc Natl Acad Sci. 2009;106:7191–7196. doi: 10.1073/pnas.0810710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bovolenta R, Zucchini S, Paradiso B, Rodi D, Merigo F, Mora GN, Osculati F, Berto E, Marconi P, Marzola A, et al. Hippocampal FGF-2 and BDNF Overexpression Attenuates Epileptogenesis-Associated Neuroinflammation and Reduces Spontaneous Recurrent Seizures. J Neuroinflammation. 2010;7:81. doi: 10.1186/1742-2094-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Paradiso B, Zucchini S, Su T, Bovolenta R, Berto E, Marconi P, Marzola A, Mora GN, Fabene PF, Simonato M. Localized Overexpression of FGF-2 and BDNF in Hippocampus Reduces Mossy Fiber Sprouting and Spontaneous Seizures up to 4 Weeks after Pilocarpine-Induced Status Epilepticus. Epilepsia. 2011;52:572–578. doi: 10.1111/j.1528-1167.2010.02930.x. [DOI] [PubMed] [Google Scholar]
- 209.Haberman RP, Samulski RJ, McCown TJ. Attenuation of Seizures and Neuronal Death by Adeno-Associated Virus Vector Galanin Expression and Secretion. Nat Med. 2003;9:1076–1080. doi: 10.1038/nm901. [DOI] [PubMed] [Google Scholar]
- 210.Lin E-JD, Richichi C, Young D, Baer K, Vezzani A, During MJ. Recombinant AAV-Mediated Expression of Galanin in Rat Hippocampus Suppresses Seizure Development. Eur J Neurosci. 2003;18:2087–2092. doi: 10.1046/j.1460-9568.2003.02926.x. [DOI] [PubMed] [Google Scholar]
- 211.McCown TJ. Adeno-Associated Virus-Mediated Expression and Constitutive Secretion of Galanin Suppresses Limbic Seizure Activity <em>in Vivo</Em>. Mol Ther. 2006;14:63–68. doi: 10.1016/j.ymthe.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 212.Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABAA Receptor Α1 Subunit Levels in Hippocampal Dentate Gyrus Inhibits Epilepsy Development in an Animal Model of Temporal Lobe Epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Drew L. Gene Therapy Targets Epilepsy. Nature. 2018;564:S10–S11. doi: 10.1038/d41586-018-07644-y. [DOI] [PubMed] [Google Scholar]
- 214.Miniarikova J, Zanella I, Huseinovic A, van der Zon T, Hanemaaijer E, Martier R, Koornneef A, Southwell AL, Hayden MR, van Deventer SJ. Design, Characterization, and Lead Selection of Therapeutic MiRNAs Targeting Huntingtin for Development of Gene Therapy for Huntington’s Disease. Mol Ther Nucleic Acids. 2016;5:297. doi: 10.1038/mtna.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Miniarikova J, Zimmer V, Martier R, Brouwers CC, Pythoud C, Richetin K, Rey M, Lubelski J, Evers MM, van Deventer SJ. AAV5-MiHTT Gene Therapy Demonstrates Suppression of Mutant Huntingtin Aggregation and Neuronal Dysfunction in a Rat Model of Huntington’s Disease. Gene Ther. 2017;24:630–639. doi: 10.1038/gt.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Evers MM, Miniarikova J, Juhas S, Vallès A, Bohuslavova B, Juhasova J, Skalnikova HK, Vodicka P, Valekova I, Brouwers C. AAV5-MiHTT Gene Therapy Demonstrates Broad Distribution and Strong Human Mutant Huntingtin Lowering in a Huntington’s Disease Minipig Model. Mol Ther. 2018;26:2163–2177. doi: 10.1016/j.ymthe.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Haque NS, Isacson O. Neurotrophic Factors NGF and FGF-2 Alter Levels of Huntingtin (IT15) in Striatal Neuronal Cell Cultures. Cell Transplant. 2000;9:623–627. doi: 10.1177/096368970000900507. [DOI] [PubMed] [Google Scholar]
- 218.de Almeida LP, Zala D, Aebischer P, Déglon N. Neuroprotective Effect of a CNTF-Expressing Lentiviral Vector in the Quinolinic Acid Rat Model of Huntington’s Disease. Neurobiol Dis. 2001;8:433–446. doi: 10.1006/nbdi.2001.0388. [DOI] [PubMed] [Google Scholar]
- 219.Ebert AD, Barber AE, Heins BM, Svendsen CN. Ex Vivo Delivery of GDNF Maintains Motor Function and Prevents Neuronal Loss in a Transgenic Mouse Model of Huntington’s Disease. Exp Neurol. 2010;224:155–162. doi: 10.1016/j.expneurol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 220.Pineda JR, Rubio N, Akerud P, Urban N, Badimon L, Arenas E, Alberch J, Blanco J, Canals JM. Neuroprotection by GDNF-Secreting Stem Cells in a Huntington’s Disease Model: Optical Neuroimage Tracking of Brain-Grafted Cells. Gene Ther. 2007;14:118–128. doi: 10.1038/sj.gt.3302847. [DOI] [PubMed] [Google Scholar]
- 221.Sado M, Yamasaki Y, Iwanaga T, Onaka Y, Ibuki T, Nishihara S, Mizuguchi H, Momota H, Kishibuchi R, Hashimoto T. Protective Effect against Parkinson’s Disease-Related Insults through the Activation of XBP1. Brain Res. 2009;1257:16–24. doi: 10.1016/j.brainres.2008.11.104. [DOI] [PubMed] [Google Scholar]
- 222.Lu X-H, Yang XW. “Huntingtin Holiday”: Progress toward an Antisense Therapy for Huntington’s Disease. Neuron. 2012;74:964–966. doi: 10.1016/j.neuron.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Matsuoka N, Nozaki K, Takagi Y, Nishimura M, Hayashi J, Miyatake S-I, Hashimoto N. Adenovirus-Mediated Gene Transfer of Fibroblast Growth Factor-2 Increases BrdU-Positive Cells after Forebrain Ischemia in Gerbils. Stroke. 2003;34:1519–1525. doi: 10.1161/01.STR.0000070840.56414.3B. [DOI] [PubMed] [Google Scholar]
- 224.Tohru K, Henrik A, Pär T, Rina K, Zaal K, Olle L. Intracerebral Infusion of Glial Cell Line-Derived Neurotrophic Factor Promotes Striatal Neurogenesis After Stroke in Adult Rats. Stroke. 2006;37:2361–2367. doi: 10.1161/01.STR.0000236025.44089.e1. [DOI] [PubMed] [Google Scholar]
- 225.Zuccato C, Cattaneo E. Brain-Derived Neurotrophic Factor in Neurodegenerative Diseases. Nat Rev Neurol. 2009;5:311. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 226.Yenari MA, Fink SL, Sun GH, Chang LK, Patel MK, Kunis DM, Onley D, Ho DY, Sapolsky RM, Steinbrg GK. Gene Therapy with HSP72 Is Neuroprotective in Rat Models of Stroke and Epilepsy. Ann Neurol. 1998;44:584–591. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]
- 227.Matthias S, Sebastian K, Stefan S, Sybille D, Werner H. Superoxide Dismutase Activity in Serum of Patients With Acute Cerebral Ischemic Injury. Stroke. 1997;28:2425–2428. doi: 10.1161/01.STR.28.12.2425. [DOI] [PubMed] [Google Scholar]
- 228.Wang Q, Liu X, Zhao J, Zhu R. Circular RNAs: Novel Diagnostic and Therapeutic Targets for Ischemic Stroke. Expert Rev Mol Diagn. 2020;20:1039–1049. doi: 10.1080/14737159.2020.1826313. [DOI] [PubMed] [Google Scholar]
- 229.Zavvarian, M.-M.; Toossi, A.; Khazaei, M.; Hong, J.; Fehlings, M. Novel Innovations in Cell and Gene Therapies for Spinal Cord Injury. F1000Research2020, 9. [DOI] [PMC free article] [PubMed]
- 230.Blanco-Ocampo D, Cawen FA, Álamo-Pindado LA, Negro-Demontel ML, Peluffo H. Safe and Neuroprotective Vectors for Long-Term Traumatic Brain Injury Gene Therapy. Gene Ther. 2020;27:96–103. doi: 10.1038/s41434-019-0073-8. [DOI] [PubMed] [Google Scholar]
- 231.Shen F, Wen L, Yang X, Liu W. The Potential Application of Gene Therapy in the Treatment of Traumatic Brain Injury. Neurosurg Rev. 2007;30:291–298. doi: 10.1007/s10143-007-0094-4. [DOI] [PubMed] [Google Scholar]
- 232.Mathew B, Parambi DGT, Mathew GE, Uddin MdS, Inasu ST, Kim H, Marathakam A, Unnikrishnan MK, Carradori S. Emerging Therapeutic Potentials of Dual-Acting MAO and AChE Inhibitors in Alzheimer’s and Parkinson’s Diseases. Arch Pharm (Weinheim) 2019;352:1900177. doi: 10.1002/ardp.201900177. [DOI] [PubMed] [Google Scholar]
- 233.Harilal S, Jose J, Parambi DGT, Kumar R, Mathew GE, Uddin MdS, Kim H, Mathew B. Advancements in Nanotherapeutics for Alzheimer’s Disease: Current Perspectives. J Pharm Pharmacol. 2019;71:1370–1383. doi: 10.1111/jphp.13132. [DOI] [PubMed] [Google Scholar]
- 234.Koliatsos VE, Nauta HJ, Clatterbuck RE, Holtzman DM, Mobley WC, Price DL. Mouse Nerve Growth Factor Prevents Degeneration of Axotomized Basal Forebrain Cholinergic Neurons in the Monkey. J. Neurosci. 1990;10:3801–3813. doi: 10.1523/JNEUROSCI.10-12-03801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kordower JH, Winn SR, Liu YT, Mufson EJ, Sladek JR, Jr, Hammang JP, Baetge EE, Emerich DF. The Aged Monkey Basal Forebrain: Rescue and Sprouting of Axotomized Basal Forebrain Neurons after Grafts of Encapsulated Cells Secreting Human Nerve Growth Factor. Proc Natl Acad Sci U S A. 1994;91:10898–10902. doi: 10.1073/pnas.91.23.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mathew B, Carradori S, Guglielmi P, Uddin MdS, Kim H. New Aspects of Monoamine Oxidase B Inhibitors: The Key Role of Halogens to Open the Golden Door. Curr Med Chem. 2020 doi: 10.2174/0929867327666200121165931. [DOI] [PubMed] [Google Scholar]
- 237.Jayakumar R, Chennazhi KP, Muzzarelli RAA, Tamura H, Nair SV, Selvamurugan N. Chitosan Conjugated DNA Nanoparticles in Gene Therapy. Carbohydr Polym. 2010;79:1–8. doi: 10.1016/j.carbpol.2009.08.026. [DOI] [Google Scholar]
- 238.Tuszynski MH, Yang JH, Barba D, Hoi-Sang U, Bakay RA, Pay MM, Masliah E, Conner JM, Kobalka P, Roy S. Nerve Growth Factor Gene Therapy: Activation of Neuronal Responses in Alzheimer Disease. JAMA Neurol. 2015;72:1139–1147. doi: 10.1001/jamaneurol.2015.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wu C-C, Lien C-C, Hou W-H, Chiang P-M, Tsai K-J. Gain of BDNF Function in Engrafted Neural Stem Cells Promotes the Therapeutic Potential for Alzheimer’s Disease. Sci Rep. 2016;6:1–16. doi: 10.1038/srep27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McGinley LM, Sims E, Lunn JS, Kashlan ON, Chen KS, Bruno ES, Pacut CM, Hazel T, Johe K, Sakowski SA. Human Cortical Neural Stem Cells Expressing Insulin-like Growth Factor-I: A Novel Cellular Therapy for Alzheimer’s Disease. Stem Cells Transl Med. 2016;5:379–391. doi: 10.5966/sctm.2015-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, Yang YS, Suh J-G, Lee B-H, Jin HK. Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Improve Neuropathology and Cognitive Impairment in an Alzheimer’s Disease Mouse Model through Modulation of Neuroinflammation. Neurobiol Aging. 2012;33:588–602. doi: 10.1016/j.neurobiolaging.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 242.Naaldijk Y, Jaeger C, Fabian C, Leovsky C, Blüher A, Rudolph L, Hinze A, Stolzing A. Effect of Systemic Transplantation of Bone Marrow-Derived Mesenchymal Stem Cells on Neuropathology Markers in APP/PS 1 Alzheimer Mice. Neuropathol Appl Neurobiol. 2017;43:299–314. doi: 10.1111/nan.12319. [DOI] [PubMed] [Google Scholar]
- 243.Kiyota T, Ingraham KL, Jacobsen MT, Xiong H, Ikezu T. FGF2 Gene Transfer Restores Hippocampal Functions in Mouse Models of Alzheimer’s Disease and Has Therapeutic Implications for Neurocognitive Disorders. Proc Natl Acad Sci. 2011;108:E1339–E1348. doi: 10.1073/pnas.1102349108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Garwood CJ, Ratcliffe LE, Simpson JE, Heath PR, Ince PG, Wharton SB. Astrocytes in Alzheimer’s Disease and Other Age-Associated Dementias: A Supporting Player with a Central Role. Neuropathol Appl Neurobiol. 2017;43:281–298. doi: 10.1111/nan.12338. [DOI] [PubMed] [Google Scholar]
- 245.Guillot-Sestier M-V, Doty KR, Town T. Innate Immunity Fights Alzheimer’s Disease. Trends Neurosci. 2015;38:674–681. doi: 10.1016/j.tins.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lauzon M-A, Daviau A, Marcos B, Faucheux N. Growth Factor Treatment to Overcome Alzheimer’s Dysfunctional Signaling. Cell Signal. 2015;27:1025–1038. doi: 10.1016/j.cellsig.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 247.Minter MR, Taylor JM, Crack PJ. The Contribution of Neuroinflammation to Amyloid Toxicity in Alzheimer’s Disease. J Neurochem. 2016;136:457–474. doi: 10.1111/jnc.13411. [DOI] [PubMed] [Google Scholar]
- 248.Namboori PKK, Vineeth KV, Rohith V, Hassan I, Sekhar L, Sekhar A, Nidheesh M. The ApoE Gene of Alzheimer’s Disease (AD) Funct Integr Genomics. 2011;11:519–522. doi: 10.1007/s10142-011-0238-z. [DOI] [PubMed] [Google Scholar]
- 249.Murphy SR, Chang CCY, Dogbevia G, Bryleva EY, Bowen Z, Hasan MT, Chang T-Y. Acat1 Knockdown Gene Therapy Decreases Amyloid-β in a Mouse Model of Alzheimer’s Disease. Mol Ther. 2013;21:1497–1506. doi: 10.1038/mt.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Saido TC. Metabolism of Amyloid β Peptide and Pathogenesis of Alzheimer’s Disease. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89:321–339. doi: 10.2183/pjab.89.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Iwata N, Sekiguchi M, Hattori Y, Takahashi A, Asai M, Ji B, Higuchi M, Staufenbiel M, Muramatsu S, Saido TC. Global Brain Delivery of Neprilysin Gene by Intravascular Administration of AAV Vector in Mice. Sci Rep. 2013;3:1472. doi: 10.1038/srep01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Carty N, Nash KR, Brownlow M, Cruite D, Wilcock D, Selenica M-LB, Lee DC, Gordon MN, Morgan D. Intracranial Injection of AAV Expressing NEP but Not IDE Reduces Amyloid Pathology in APP+PS1 Transgenic Mice. PLOS ONE. 2013;8:e59626. doi: 10.1371/journal.pone.0059626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Marwarha G, Dasari B, Prasanthi JRP, Schommer J, Ghribi O. Leptin Reduces the Accumulation of Abeta and Phosphorylated Tau Induced by 27-Hydroxycholesterol in Rabbit Organotypic Slices. J Alzheimers Dis JAD. 2010;19:1007–1019. doi: 10.3233/JAD-2010-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhang Q, Li N, Jiao X, Qin X, Kaur R, Lu X, Song J, Wang L, Wang J, Niu Q. Caspase-3 Short Hairpin RNAs: A Potential Therapeutic Agent in Neurodegeneration of Aluminum-Exposed Animal Model. Curr Alzheimer Res. 2014;11(10):961–970. doi: 10.2174/1567205011666141107150938. [DOI] [PubMed] [Google Scholar]
- 255.Chai X, Kong W, Liu L, Yu W, Zhang Z, Sun Y. A Viral Vector Expressing Hypoxia-Inducible Factor 1 Alpha Inhibits Hippocampal Neuronal Apoptosis. Neural Regen Res. 2014;9:1145–1153. doi: 10.4103/1673-5374.135317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc J-L. Limitations of Current Parkinson’s Disease Therapy. Ann Neurol. 2003;53:S3–S15. doi: 10.1002/ana.10513. [DOI] [PubMed] [Google Scholar]
- 257.Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, III, Gasmi M, Bartus RT. Delivery of Neurturin by AAV2 (CERE-120)-Mediated Gene Transfer Provides Structural and Functional Neuroprotection and Neurorestoration in MPTP-Treated Monkeys. Ann Neurol Off J Am Neurol Assoc Child Neurol Soc. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- 258.Dass B, Olanow CW, Kordower JH. Gene Transfer of Trophic Factors and Stem Cell Grafting as Treatments for Parkinson’s Disease. Neurology. 2006;66:S89–S103. doi: 10.1212/WNL.66.10_suppl_4.S89. [DOI] [PubMed] [Google Scholar]
- 259.Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P. Randomized Controlled Trial of Intraputamenal Glial Cell Line-Derived Neurotrophic Factor Infusion in Parkinson Disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 260.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen E-Y, Palfi S, Roitberg BZ. Neurodegeneration Prevented by Lentiviral Vector Delivery of GDNF in Primate Models of Parkinson’s Disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 261.Zuo F-X, Bao X-J, Sun X-C, Wu J, Bai Q-R, Chen G, Li X-Y, Zhou Q-Y, Yang Y-F, Shen Q. Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment. Int J Mol Sci. 2015;16:26473–26492. doi: 10.3390/ijms161125966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ebert AD, Beres AJ, Barber AE, Svendsen CN. Human Neural Progenitor Cells Over-Expressing IGF-1 Protect Dopamine Neurons and Restore Function in a Rat Model of Parkinson’s Disease. Exp Neurol. 2008;209:213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 263.Rumpel R, Hohmann M, Klein A, Wesemann M, Baumgärtner W, Ratzka A, Grothe C. Transplantation of Fetal Ventral Mesencephalic Progenitor Cells Overexpressing High Molecular Weight Fibroblast Growth Factor 2 Isoforms in 6-Hydroxydopamine Lesioned Rats. Neuroscience. 2015;286:293–307. doi: 10.1016/j.neuroscience.2014.11.060. [DOI] [PubMed] [Google Scholar]
- 264.Glavaski-Joksimovic A, Bohn MC. Mesenchymal Stem Cells and Neuroregeneration in Parkinson’s Disease. Exp Neurol. 2013;247:25–38. doi: 10.1016/j.expneurol.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 265.Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct Brain Infusion of Glial Cell Line-Derived Neurotrophic Factor in Parkinson Disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 266.Gash DM, Kryscio R, Young B. Research on Parkinson Disease. J Neurosurg. 2005;102:401. [PubMed] [Google Scholar]
- 267.Hovland DN, Boyd RB, Butt MT, Engelhardt JA, Moxness MS, Ma MH, Emery MG, Ernst NB, Reed RP, Zeller JR, et al. Six-Month Continuous Intraputamenal Infusion Toxicity Study of Recombinant Methionyl Human Glial Cell Line-Derived Neurotrophic Factor (r-MetHuGDNF) in Rhesus Monkeys. Toxicol Pathol. 2007;35:676–692. doi: 10.1177/01926230701481899a. [DOI] [PubMed] [Google Scholar]
- 268.Manfredsson FP, Burger C, Rising AC, Zuobi-Hasona K, Sullivan LF, Lewin AS, Huang J, Piercefield E, Muzyczka N, Mandel RJ. Tight Long-Term Dynamic Doxycycline Responsive Nigrostriatal GDNF Using a Single RAAV Vector. Mol Ther. 2009;17:1857–1867. doi: 10.1038/mt.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rosenblad C, Georgievska B, Kirik D. Long-Term Striatal Overexpression of GDNF Selectively Downregulates Tyrosine Hydroxylase in the Intact Nigrostriatal Dopamine System. Eur J Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- 270.Quintino L, Manfré G, Wettergren EE, Namislo A, Isaksson C, Lundberg C. Functional Neuroprotection and Efficient Regulation of GDNF Using Destabilizing Domains in a Rodent Model of Parkinson’s Disease. Mol Ther J Am Soc Gene Ther. 2013;21:2169–2180. doi: 10.1038/mt.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Iwamoto M, Björklund T, Lundberg C, Kirik D, Wandless TJ. A General Chemical Method to Regulate Protein Stability in the Mammalian Central Nervous System. Chem Biol. 2010;17:981–988. doi: 10.1016/j.chembiol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bartus RT, Brown L, Wilson A, Kruegel B, Siffert J, Johnson EM, Kordower JH, Herzog CD. Properly Scaled and Targeted AAV2-NRTN (Neurturin) to the Substantia Nigra Is Safe, Effective and Causes No Weight Loss: Support for Nigral Targeting in Parkinson’s Disease. Neurobiol Dis. 2011;44:38–52. doi: 10.1016/j.nbd.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 273.Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B. Long-Term Clinical Improvement in MPTP-Lesioned Primates after Gene Therapy with AAV-HAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 274.Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS. Eight Years of Clinical Improvement in MPTP-Lesioned Primates after Gene Therapy with AAV2-HAADC. Mol Ther. 2010;18:1458–1461. doi: 10.1038/mt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Forsayeth JR, Eberling JL, Sanftner LM, Zhen Z, Pivirotto P, Bringas J, Cunningham J, Bankiewicz KS. A Dose-Ranging Study of AAV-HAADC Therapy in Parkinsonian Monkeys. Mol Ther. 2006;14:571–577. doi: 10.1016/j.ymthe.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.San Sebastian W, Richardson RM, Kells AP, Lamarre C, Bringas J, Pivirotto P, Salegio EA, DeArmond SJ, Forsayeth J, Bankiewicz KS. Safety and Tolerability of Magnetic Resonance Imaging-Guided Convection-Enhanced Delivery of AAV2-HAADC with a Novel Delivery Platform in Nonhuman Primate Striatum. Hum Gene Ther. 2012;23:210–217. doi: 10.1089/hum.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. Convection-Enhanced Delivery of AAV Vector in Parkinsonian Monkeys; in Vivo Detection of Gene Expression and Restoration of Dopaminergic Function Using pro-Drug Approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 278.Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, Rohll JB, Kingsman SM, Kingsman AJ, Mazarakis ND. Multicistronic Lentiviral Vector-Mediated Striatal Gene Transfer of Aromatic L-Amino Acid Decarboxylase, Tyrosine Hydroxylase, and GTP Cyclohydrolase I Induces Sustained Transgene Expression, Dopamine Production, and Functional Improvement in a Rat Model. J Neurosci Off J Soc Neurosci. 2002;22:10302–10312. doi: 10.1523/JNEUROSCI.22-23-10302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, Kaplan PL, Forsayeth J, Aminoff MJ, Bankiewicz KS. Long-Term Evaluation of a Phase 1 Study of AADC Gene Therapy for Parkinson’s Disease. Hum Gene Ther. 2012;23:377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sebastian WS, Kells AP, Bringas J, Samaranch L, Hadaczek P, Ciesielska A, Macayan MJ, Pivirotto PJ, Forsayeth J, Osborne S, et al. Safety and Tolerability of MRI-Guided Infusion of AAV2-HAADC into the Mid-Brain of Nonhuman Primate. Mol. Ther. - Methods Clin. Dev. 2014;3:14049. doi: 10.1038/mtm.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Cederfjäll E, Sahin G, Kirik D, Björklund T. Design of a Single AAV Vector for Coexpression of TH and GCH1 to Establish Continuous DOPA Synthesis in a Rat Model of Parkinson’s Disease. Mol Ther J Am Soc Gene Ther. 2012;20:1315–1326. doi: 10.1038/mt.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ren X, Zhang T, Gong X, Hu G, Ding W, Wang X. AAV2-Mediated Striatum Delivery of Human CDNF Prevents the Deterioration of Midbrain Dopamine Neurons in a 6-Hydroxydopamine Induced Parkinsonian Rat Model. Exp Neurol. 2013;248:148–156. doi: 10.1016/j.expneurol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 283.Georgievska B, Kirik D, Björklund A. Aberrant Sprouting and Downregulation of Tyrosine Hydroxylase in Lesioned Nigrostriatal Dopamine Neurons Induced by Long-Lasting Overexpression of Glial Cell Line Derived Neurotrophic Factor in the Striatum by Lentiviral Gene Transfer. Exp Neurol. 2002;177:461–474. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- 284.Bäck S, Peränen J, Galli E, Pulkkila P, Lonka-Nevalaita L, Tamminen T, Voutilainen MH, Raasmaja A, Saarma M, Männistö PT, et al. Gene Therapy with AAV2-CDNF Provides Functional Benefits in a Rat Model of Parkinson’s Disease. Brain Behav. 2013;3:75–88. doi: 10.1002/brb3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Leblond CS, Kaneb HM, Dion PA, Rouleau GA. Dissection of Genetic Factors Associated with Amyotrophic Lateral Sclerosis. Exp Neurol. 2014;262:91–101. doi: 10.1016/j.expneurol.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 286.Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, Andres PL, Mahoney K, Allred P, Alexander K, et al. An Antisense Oligonucleotide against SOD1 Delivered Intrathecally for Patients with SOD1 Familial Amyotrophic Lateral Sclerosis: A Phase 1, Randomised, First-in-Man Study. Lancet Neurol. 2013;12:435–442. doi: 10.1016/S1474-4422(13)70061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Aebischer P, Schluep M, Déglon N, Joseph J-M, Hirt L, Heyd B, Goddard M, Hammang JP, Zurn AD, Kato AC. Intrathecal Delivery of CNTF Using Encapsulated Genetically Modifiedxenogeneic Cells in Amyotrophic Lateral Sclerosis Patients. Nat Med. 1996;2:696–699. doi: 10.1038/nm0696-696. [DOI] [PubMed] [Google Scholar]
- 288.Kaspar BK. Mesenchymal Stem Cells as Trojan Horses for GDNF Delivery in ALS. Mol Ther. 2008;16:1905–1906. doi: 10.1038/mt.2008.216. [DOI] [PubMed] [Google Scholar]
- 289.Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, Bellantuono I, Aebischer P, Svendsen CN. Direct Muscle Delivery of GDNF with Human Mesenchymal Stem Cells Improves Motor Neuron Survival and Function in a Rat Model of Familial ALS. Mol Ther. 2008;16:2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, Bellantuono I, Aebischer P, Svendsen CN. Intramuscular Delivery of GDNF with Human Mesenchymal Stem Cells Protects Dying Motor Neurons and Prolongs Survival in a Rat Model of Familial ALS. Cell Transplant. 2008;17:482–483. [Google Scholar]
- 291.Acsadi G, Anguelov RA, Yang H, Toth G, Thomas R, Jani A, Wang Y, Ianakova E, Mohammad S, Lewis RA. Increased Survival and Function of SOD1 Mice after Glial Cell-Derived Neurotrophic Factor Gene Therapy. Hum Gene Ther. 2002;13:1047–1059. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- 292.Haase G, Kennel P, Pettmann B, Vigne E, Akli S, Revah F, Schmalbruch H, Kahn A. Gene Therapy of Murine Motor Neuron Disease Using Adenoviral Vectors for Neurotrophic Factors. Nat Med. 1997;3:429–436. doi: 10.1038/nm0497-429. [DOI] [PubMed] [Google Scholar]
- 293.Boillée S, Cleveland DW. Gene Therapy for ALS Delivers. Trends Neurosci. 2004;27:235–238. doi: 10.1016/j.tins.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 294.Deshpande DM, Kim Y-S, Martinez T, Carmen J, Dike S, Shats I, Rubin LL, Drummond J, Krishnan C, Hoke A. Recovery from Paralysis in Adult Rats Using Embryonic Stem Cells. Ann Neurol. 2006;60:32–44. doi: 10.1002/ana.20901. [DOI] [PubMed] [Google Scholar]
- 295.Harper JM, Krishnan C, Darman JS, Deshpande DM, Peck S, Shats I, Backovic S, Rothstein JD, Kerr DA. Axonal Growth of Embryonic Stem Cell-Derived Motoneurons in Vitro and in Motoneuron-Injured Adult Rats. Proc Natl Acad Sci. 2004;101:7123–7128. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.López-González R, Kunckles P, Velasco I. Transient Recovery in a Rat Model of Familial Amyotrophic Lateral Sclerosis after Transplantation of Motor Neurons Derived from Mouse Embryonic Stem Cells. Cell Transplant. 2009;18:1171–1181. doi: 10.3727/096368909X12483162197123. [DOI] [PubMed] [Google Scholar]
- 297.Yohn DC, Miles GB, Rafuse VF, Brownstone RM. Transplanted Mouse Embryonic Stem-Cell-Derived Motoneurons Form Functional Motor Units and Reduce Muscle Atrophy. J Neurosci. 2008;28:12409–12418. doi: 10.1523/JNEUROSCI.1761-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gowing G, Svendsen CN. Stem Cell Transplantation for Motor Neuron Disease: Current Approaches and Future Perspectives. Neurotherapeutics. 2011;8:591–606. doi: 10.1007/s13311-011-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Knippenberg S, Rath KJ, Böselt S, Thau-Habermann N, Schwarz SC, Dengler R, Wegner F, Petri S. Intraspinal Administration of Human Spinal Cord-Derived Neural Progenitor Cells in the G93A–SOD1 Mouse Model of ALS Delays Symptom Progression, Prolongs Survival and Increases Expression of Endogenous Neurotrophic Factors. J Tissue Eng Regen Med. 2017;11:751–764. doi: 10.1002/term.1972. [DOI] [PubMed] [Google Scholar]
- 300.Kondo T, Funayama M, Tsukita K, Hotta A, Yasuda A, Nori S, Kaneko S, Nakamura M, Takahashi R, Okano H. Focal Transplantation of Human IPSC-Derived Glial-Rich Neural Progenitors Improves Lifespan of ALS Mice. Stem Cell Rep. 2014;3:242–249. doi: 10.1016/j.stemcr.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Park S, Kim H-T, Yun S, Kim I-S, Lee J, Lee I-S, Park KI. Growth Factor-Expressing Human Neural Progenitor Cell Grafts Protect Motor Neurons but Do Not Ameliorate Motor Performance and Survival in ALS Mice. Exp Mol Med. 2009;41:487–500. doi: 10.3858/emm.2009.41.7.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN. Intermittent Hypoxia and Stem Cell Implants Preserve Breathing Capacity in a Rodent Model of Amyotrophic Lateral Sclerosis. Am J Respir Crit Care Med. 2013;187:535–542. doi: 10.1164/rccm.201206-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lewis CM, Suzuki M. Therapeutic Applications of Mesenchymal Stem Cells for Amyotrophic Lateral Sclerosis. Stem Cell Res Ther. 2014;5:1–10. doi: 10.1186/scrt421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Petrou P, Gothelf Y, Argov Z, Gotkine M, Levy YS, Kassis I, Vaknin-Dembinsky A, Ben-Hur T, Offen D, Abramsky O. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients with Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016;73:337–344. doi: 10.1001/jamaneurol.2015.4321. [DOI] [PubMed] [Google Scholar]
- 305.Thomsen GM, Gowing G, Latter J, Chen M, Vit J-P, Staggenborg K, Avalos P, Alkaslasi M, Ferraiuolo L, Likhite S, et al. Delayed Disease Onset and Extended Survival in the SOD1G93A Rat Model of Amyotrophic Lateral Sclerosis after Suppression of Mutant SOD1 in the Motor Cortex. J Neurosci Off J Soc Neurosci. 2014;34:15587–15600. doi: 10.1523/JNEUROSCI.2037-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Patel P, Kriz J, Gravel M, Soucy G, Bareil C, Gravel C, Julien J-P. Adeno-Associated Virus-Mediated Delivery of a Recombinant Single-Chain Antibody against Misfolded Superoxide Dismutase for Treatment of Amyotrophic Lateral Sclerosis. Mol Ther J Am Soc Gene Ther. 2014;22:498–510. doi: 10.1038/mt.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, Rao M, Eagle A, Matthews JC, Taksir TV, et al. AAV4-Mediated Expression of IGF-1 and VEGF within Cellular Components of the Ventricular System Improves Survival Outcome in Familial ALS Mice. Mol Ther J Am Soc Gene Ther. 2010;18:2075–2084. doi: 10.1038/mt.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kliem MA, Heeke BL, Franz CK, Radovitskiy I, Raore B, Barrow E, Snyder BR, Federici T, Kaye Spratt S, Boulis NM. Intramuscular Administration of a VEGF Zinc Finger Transcription Factor Activator (VEGF-ZFP-TF) Improves Functional Outcomes in SOD1 Rats. Amyotroph Lateral Scler. 2011;12:331–339. doi: 10.3109/17482968.2011.574142. [DOI] [PubMed] [Google Scholar]
- 309.Henriques A, Pitzer C, Dittgen T, Klugmann M, Dupuis L, Schneider A. CNS-Targeted Viral Delivery of G-CSF in an Animal Model for ALS: Improved Efficacy and Preservation of the Neuromuscular Unit. Mol Ther J Am Soc Gene Ther. 2011;19:284–292. doi: 10.1038/mt.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Johnson JO, Pioro EP, Boehringer A, Chia R, Feit H, Renton AE, Pliner HA, Abramzon Y, Marangi G, Winborn BJ, et al. Mutations in the Matrin 3 Gene Cause Familial Amyotrophic Lateral Sclerosis. Nat Neurosci. 2014;17:664–666. doi: 10.1038/nn.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Baxter S, Reed H, Clarke Z, Judge S, Heron N, Mccarthy A, Langley J, Stanton A, Wells O, Squire G, et al. Evaluating a Novel Cervical Orthosis, the Sheffield Support Snood, in Patients with Amyotrophic Lateral Sclerosis/Motor Neuron Disease with Neck Weakness. Amyotroph Lateral Scler Front Degener. 2016;17:436–442. doi: 10.3109/21678421.2016.1148170. [DOI] [PubMed] [Google Scholar]
- 312.Meininger V, Genge A, van den Berg LH, Robberecht W, Ludolph A, Chio A, Kim SH, Leigh PN, Kiernan MC, Shefner JM, et al. Safety and Efficacy of Ozanezumab in Patients with Amyotrophic Lateral Sclerosis: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2017;16:208–216. doi: 10.1016/s1474-4422(16)30399-4. [DOI] [PubMed] [Google Scholar]
- 313.Nicaise C, Mitrecic D, Pochet R. Brain and Spinal Cord Affected by Amyotrophic Lateral Sclerosis Induce Differential Growth Factors Expression in Rat Mesenchymal and Neural Stem Cells. Neuropathol Appl Neurobiol. 2011;37:179–188. doi: 10.1111/j.1365-2990.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- 314.Faravelli I, Nizzardo M, Comi GP, Corti S. Spinal Muscular Atrophy—Recent Therapeutic Advances for an Old Challenge. Nat Rev Neurol. 2015;11:351–359. doi: 10.1038/nrneurol.2015.77. [DOI] [PubMed] [Google Scholar]
- 315.Donnelly EM, Boulis NM. Update on Gene and Stem Cell Therapy Approaches for Spinal Muscular Atrophy. Expert Opin Biol Ther. 2012;12:1463–1471. doi: 10.1517/14712598.2012.711306. [DOI] [PubMed] [Google Scholar]
- 316.Bevan AK, Hutchinson KR, Foust KD, Braun L, McGovern VL, Schmelzer L, Ward JG, Petruska JC, Lucchesi PA, Burghes AHM, et al. Early Heart Failure in the SMNDelta7 Model of Spinal Muscular Atrophy and Correction by Postnatal ScAAV9-SMN Delivery. Hum Mol Genet. 2010;19:3895–3905. doi: 10.1093/hmg/ddq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Duque SI, Arnold WD, Odermatt P, Li X, Porensky PN, Schmelzer L, Meyer K, Kolb SJ, Schümperli D, Kaspar BK, et al. A Large Animal Model of Spinal Muscular Atrophy and Correction of Phenotype. Ann Neurol. 2015;77:399–414. doi: 10.1002/ana.24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Afonso-Reis R, Afonso IT, Nóbrega C. Current Status of Gene Therapy Research in Polyglutamine Spinocerebellar Ataxias. Int J Mol Sci. 2021;22:4249. doi: 10.3390/ijms22084249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tan S, Wang R-H, Niu H-X, Shi C-H, Mao C-Y, Zhang R, Song B, Sun S-L, Liu X-J, Hou H-M. Nerve Growth Factor for the Treatment of Spinocerebellar Ataxia Type 3: An Open-Label Study. Chin Med J (Engl) 2015;128:291. doi: 10.4103/0366-6999.150087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chen Y-S, Hong Z-X, Lin S-Z, Harn H-J. Identifying Therapeutic Targets for Spinocerebellar Ataxia Type 3/Machado–Joseph Disease through Integration of Pathological Biomarkers and Therapeutic Strategies. Int J Mol Sci. 2020;21:3063. doi: 10.3390/ijms21093063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Fisher RS, van Boas WE, Blume W, Elger C, Genton P, Lee P, Jr, Engel J. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 322.Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABA<Sub>A</Sub> Receptor Α1 Subunit Levels in Hippocampal Dentate Gyrus Inhibits Epilepsy Development in an Animal Model of Temporal Lobe Epilepsy. J. Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Richichi C, Lin E-JD, Stefanin D, Colella D, Ravizza T, Grignaschi G, Veglianese P, Sperk G, During MJ, Vezzani A. Anticonvulsant and Antiepileptogenic Effects Mediated by Adeno-Associated Virus Vector Neuropeptide Y Expression in the Rat Hippocampus. J. Neurosci. 2004;24:3051–3059. doi: 10.1523/JNEUROSCI.4056-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Foti S, Haberman RP, Samulski RJ, McCown TJ. Adeno-Associated Virus-Mediated Expression and Constitutive Secretion of NPY or NPY13-36 Suppresses Seizure Activity in Vivo. Gene Ther. 2007;14:1534–1536. doi: 10.1038/sj.gt.3303013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Noè F, Pool A-H, Nissinen J, Gobbi M, Bland R, Rizzi M, Balducci C, Ferraguti F, Sperk G, During MJ, et al. Neuropeptide Y Gene Therapy Decreases Chronic Spontaneous Seizures in a Rat Model of Temporal Lobe Epilepsy. Brain. 2008;131:1506–1515. doi: 10.1093/brain/awn079. [DOI] [PubMed] [Google Scholar]
- 326.Sørensen AT, Nikitidou L, Ledri M, Lin E-JD, During MJ, Kanter-Schlifke I, Kokaia M. Hippocampal NPY Gene Transfer Attenuates Seizures without Affecting Epilepsy-Induced Impairment of LTP. Exp Neurol. 2009;215:328–333. doi: 10.1016/j.expneurol.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Noe F, Vaghi V, Balducci C, Fitzsimons H, Bland R, Zardoni D, Sperk G, Carli M, During MJ, Vezzani A. Anticonvulsant Effects and Behavioural Outcomes of RAAV Serotype 1 Vector-Mediated Neuropeptide Y Overexpression in Rat Hippocampus. Gene Ther. 2010;17:643–652. doi: 10.1038/gt.2010.23. [DOI] [PubMed] [Google Scholar]
- 328.Woldbye DPD, Ängehagen M, Gøtzsche CR, Elbrønd-Bek H, Sørensen AT, Christiansen SH, Olesen MV, Nikitidou L, Hansen TVO, Kanter-Schlifke I, et al. Adeno-Associated Viral Vector-Induced Overexpression of Neuropeptide Y Y2 Receptors in the Hippocampus Suppresses Seizures. Brain. 2010;133:2778–2788. doi: 10.1093/brain/awq219. [DOI] [PubMed] [Google Scholar]
- 329.Lieb A, Qiu Y, Dixon CL, Heller JP, Walker MC, Schorge S, Kullmann DM. Biochemical Autoregulatory Gene Therapy for Focal Epilepsy. Nat Med. 2018;24:1324–1329. doi: 10.1038/s41591-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.(2020) University College, London Phase I/IIa, First-in-Human, Open-Label, Single-Site Trial of In-Vivo Lentiviral Engineered Potassium (K+) Channel (EKC) Gene Therapy for Refractory Epilepsy; clinicaltrials.gov
- 331.MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, Barnes G, Taylor SA, James M, Groot N, et al. A Novel Gene Containing a Trinucleotide Repeat That Is Expanded and Unstable on Huntington’s Disease Chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 332.Bao J, Sharp AH, Wagster MV, Becher M, Schilling G, Ross CA, Dawson VL, Dawson TM. Expansion of Polyglutamine Repeat in Huntingtin Leads to Abnormal Protein Interactions Involving Calmodulin. Proc Natl Acad Sci U S A. 1996;93:5037–5042. doi: 10.1073/pnas.93.10.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Bemelmans A-P, Horellou P, Pradier L, Brunet I, Colin P, Mallet J. Brain-Derived Neurotrophic Factor-Mediated Protection of Striatal Neurons in an Excitotoxic Rat Model of Huntington’s Disease, as Demonstrated by Adenoviral Gene Transfer. Hum Gene Ther. 1999;10:2987–2997. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- 334.Fink KD, Deng P, Torrest A, Stewart H, Pollock K, Gruenloh W, Annett G, Tempkin T, Wheelock V, Nolta JA. Developing Stem Cell Therapies for Juvenile and Adult-Onset Huntington’s Disease. Regen Med. 2015;10:623–646. doi: 10.2217/rme.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Puigdellívol M, Saavedra A, Pérez-Navarro E. Cognitive Dysfunction in H Untington’s Disease: Mechanisms and Therapeutic Strategies beyond BDNF. Brain Pathol. 2016;26:752–771. doi: 10.1111/bpa.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Samaranch L, Blits B, San Sebastian W, Hadaczek P, Bringas J, Sudhakar V, Macayan M, Pivirotto PJ, Petry H, Bankiewicz KS. MR-Guided Parenchymal Delivery of Adeno-Associated Viral Vector Serotype 5 in Non-Human Primate Brain. Gene Ther. 2017;24:253–261. doi: 10.1038/gt.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Southwell AL, Ko J, Patterson PH. Intrabody Gene Therapy Ameliorates Motor, Cognitive, and Neuropathological Symptoms in Multiple Mouse Models of Huntington’s Disease. J Neurosci Off J Soc Neurosci. 2009;29:13589–13602. doi: 10.1523/JNEUROSCI.4286-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 Overexpression Protects against Neuron Loss within the Ischemic Margin Following Experimental Stroke and Inhibits Cytochrome c Translocation and Caspase-3 Activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 339.Chang D-J, Lee N, Choi C, Jeon I, Oh S-H, Shin DA, Hwang T-S, Lee HJ, Kim SU, Moon H. Therapeutic Effect of BDNF-Overexpressing Human Neural Stem Cells (HB1. F3. BDNF) in a Rodent Model of Middle Cerebral Artery Occlusion. Cell Transplant. 2013;22:1441–1452. doi: 10.3727/096368912X657323. [DOI] [PubMed] [Google Scholar]
- 340.Chen B, Gao X-Q, Yang C-X, Tan S-K, Sun Z-L, Yan N-H, Pang Y-G, Yuan M, Chen G-J, Xu G-T. Neuroprotective Effect of Grafting GDNF Gene-Modified Neural Stem Cells on Cerebral Ischemia in Rats. Brain Res. 2009;1284:1–11. doi: 10.1016/j.brainres.2009.05.100. [DOI] [PubMed] [Google Scholar]
- 341.Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous Administration of Glial Cell Line-Derived Neurotrophic Factor Gene-Modified Human Mesenchymal Stem Cells Protects against Injury in a Cerebral Ischemia Model in the Adult Rat. J Neurosci Res. 2006;84:1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y. Mesenchymal Stem Cells That Produce Neurotrophic Factors Reduce Ischemic Damage in the Rat Middle Cerebral Artery Occlusion Model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 343.Miki Y, Nonoguchi N, Ikeda N, Coffin RS, Kuroiwa T, Miyatake S-I. Vascular Endothelial Growth Factor Gene-Transferred Bone Marrow Stromal Cells Engineered with a Herpes Simplex Virus Type 1 Vector Can Improve Neurological Deficits and Reduce Infarction Volume in Rat Brain Ischemia. Neurosurgery. 2007;61:586–595. doi: 10.1227/01.NEU.0000290907.30814.42. [DOI] [PubMed] [Google Scholar]
- 344.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL. Human Neural Stem Cells Enhance Structural Plasticity and Axonal Transport in the Ischaemic Brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Popa-Wagner A, Filfan M, Uzoni A, Pourgolafshan P, Buga A-M. Poststroke Cell Therapy of the Aged Brain. Neural Plast. 2015;2015:8396. doi: 10.1155/2015/839638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 347.Nimura T, Weinstein PR, Massa SM, Panter S, Sharp FR. Heme Oxygenase-1 (HO-1) Protein Induction in Rat Brain Following Focal Ischemia. Mol Brain Res. 1996;37:201–208. doi: 10.1016/0169-328X(95)00315-J. [DOI] [PubMed] [Google Scholar]
- 348.Hoehn B, Yenari MA, Sapolsky RM, Steinberg GK. Glutathione Peroxidase Overexpression Inhibits Cytochrome C Release and Proapoptotic Mediators to Protect Neurons from Experimental Stroke. Stroke. 2003;34:2489–2494. doi: 10.1161/01.str.0000091268.25816.19. [DOI] [PubMed] [Google Scholar]
- 349.Wang J, Xia J, Zhang F, Shi Y, Wu Y, Pu H, Liou AK, Leak RK, Yu X, Chen L. Galectin-1-Secreting Neural Stem Cells Elicit Long-Term Neuroprotection against Ischemic Brain Injury. Sci Rep. 2015;5:1–10. doi: 10.1038/srep09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sakata H, Niizuma K, Wakai T, Narasimhan P, Maier CM, Chan PH. Neural Stem Cells Genetically Modified to Overexpress Cu/Zn-Superoxide Dismutase Enhance Amelioration of Ischemic Stroke in Mice. Stroke. 2012;43:2423–2429. doi: 10.1161/STROKEAHA.112.656900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJA. Decorin Suppresses Neurocan, Brevican, Phosphacan and NG2 Expression and Promotes Axon Growth across Adult Rat Spinal Cord Injuries. Eur J Neurosci. 2004;19:1226–1242. doi: 10.1111/j.1460-9568.2004.03184.x. [DOI] [PubMed] [Google Scholar]
- 352.Tokumine J, Kakinohana O, Cizkova D, Smith DW, Marsala M. Changes in Spinal GDNF, BDNF, and NT-3 Expression after Transient Spinal Cord Ischemia in the Rat. J Neurosci Res. 2003;74:552–561. doi: 10.1002/jnr.10760. [DOI] [PubMed] [Google Scholar]
- 353.Papastefanaki F, Chen J, Lavdas AA, Thomaidou D, Schachner M, Matsas R. Grafts of Schwann Cells Engineered to Express PSA-NCAM Promote Functional Recovery after Spinal Cord Injury. Brain. 2007;130:2159–2174. doi: 10.1093/brain/awm155. [DOI] [PubMed] [Google Scholar]
- 354.Yamane J, Nakamura M, Iwanami A, Sakaguchi M, Katoh H, Yamada M, Momoshima S, Miyao S, Ishii K, Tamaoki N, et al. Transplantation of Galectin-1-Expressing Human Neural Stem Cells into the Injured Spinal Cord of Adult Common Marmosets. J Neurosci Res. 2010;88:1394–1405. doi: 10.1002/jnr.22322. [DOI] [PubMed] [Google Scholar]
- 355.White RE, Yin FQ, Jakeman LB. TGF-Alpha Increases Astrocyte Invasion and Promotes Axonal Growth into the Lesion Following Spinal Cord Injury in Mice. Exp Neurol. 2008;214:10–24. doi: 10.1016/j.expneurol.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.King VR, Alovskaya A, Wei DYT, Brown RA, Priestley J. V The Use of Injectable Forms of Fibrin and Fibronectin to Support Axonal Ingrowth after Spinal Cord Injury. Biomaterials. 2010;31:4447–4456. doi: 10.1016/j.biomaterials.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 357.Cheng H, Huang Y-C, Chang P-T, Huang Y-Y. Laminin-Incorporated Nerve Conduits Made by Plasma Treatment for Repairing Spinal Cord Injury. Biochem Biophys Res Commun. 2007;357:938–944. doi: 10.1016/j.bbrc.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 358.Lander AD, Fujii DK, Gospodarowicz D, Reichardt LF. Characterization of a Factor That Promotes Neurite Outgrowth: Evidence Linking Activity to a Heparan Sulfate Proteoglycan. J Cell Biol. 1982;94:574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Kerr BJ, Patterson PH. Leukemia Inhibitory Factor Promotes Oligodendrocyte Survival after Spinal Cord Injury. Glia. 2005;51:73–79. doi: 10.1002/glia.20177. [DOI] [PubMed] [Google Scholar]
- 360.Jones TB, Ankeny DP, Guan Z, McGaughy V, Fisher LC, Basso DM, Popovich PG. Passive or Active Immunization with Myelin Basic Protein Impairs Neurological Function and Exacerbates Neuropathology after Spinal Cord Injury in Rats. J Neurosci Off J Soc Neurosci. 2004;24:3752–3761. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Saatman KE, Duhaime A-C, Bullock R, Maas AI, Valadka A, Manley GT. Classification of Traumatic Brain Injury for Targeted Therapies. J Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, Zetterberg H. Traumatic Brain Injuries. Nat Rev Dis Primer. 2016;2:1–19. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- 363.Feng L, Chao J, Tian E, Li L, Ye P, Zhang M, Chen X, Cui Q, Sun G, Zhou T. Cell-Based Therapy for Canavan Disease Using Human IPSC-Derived NPCs and OPCs. Adv Sci. 2020;7:2002155. doi: 10.1002/advs.202002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Parenti G, Medina DL, Ballabio A. The Rapidly Evolving View of Lysosomal Storage Diseases. EMBO Mol. Med. 2021;13:e12836. doi: 10.15252/emmm.202012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Berger J, Gärtner J. X-Linked Adrenoleukodystrophy: Clinical, Biochemical and Pathogenetic Aspects. Biochim Biophys Acta BBA-Mol Cell Res. 2006;1763:1721–1732. doi: 10.1016/j.bbamcr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 366.Biffi A, Aubourg P, Cartier N. Gene Therapy for Leukodystrophies. Hum Mol Genet. 2011;20:R42–R53. doi: 10.1093/hmg/ddr142. [DOI] [PubMed] [Google Scholar]
- 367.Berger J, Forss-Petter S, Eichler FS. Pathophysiology of X-Linked Adrenoleukodystrophy. Biochimie. 2014;98:135–142. doi: 10.1016/j.biochi.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Berger J, Pujol A, Aubourg P, Forss-Petter S. Current and Future Pharmacological Treatment Strategies in X-Linked Adrenoleukodystrophy. Brain Pathol. 2010;20:845–856. doi: 10.1111/j.1750-3639.2010.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Weng S-M, Bailey ME, Cobb SR. Rett Syndrome: From Bed to Bench. Pediatr Neonatol. 2011;52:309–316. doi: 10.1016/j.pedneo.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 370.Castro J, Garcia RI, Kwok S, Banerjee A, Petravicz J, Woodson J, Mellios N, Tropea D, Sur M. Functional Recovery with Recombinant Human IGF1 Treatment in a Mouse Model of Rett Syndrome. Proc Natl Acad Sci. 2014;111:9941–9946. doi: 10.1073/pnas.1311685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Subramaniam, V. AVXS-201 - Rett Syndrome News.
- 372.Paul S, Bravo Vázquez LA, Pérez Uribe S, Roxana Reyes-Pérez P, Sharma A. Current Status of MicroRNA-Based Therapeutic Approaches in Neurodegenerative Disorders. Cells. 2020;9:1698. doi: 10.3390/cells9071698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Duarte F, Déglon N. Genome Editing for CNS Disorders. Front Neurosci. 2020;14:1039. doi: 10.3389/fnins.2020.579062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Karimian A, Gorjizadeh N, Alemi F, Asemi Z, Azizian K, Soleimanpour J, Malakouti F, Targhazeh N, Majidinia M, Yousefi B. CRISPR/Cas9 Novel Therapeutic Road for the Treatment of Neurodegenerative Diseases. Life Sci. 2020;259:118165. doi: 10.1016/j.lfs.2020.118165. [DOI] [PubMed] [Google Scholar]
- 375.Park H, Oh J, Shim G, Cho B, Chang Y, Kim S, Baek S, Kim H, Shin J, Choi H. In Vivo Neuronal Gene Editing via CRISPR–Cas9 Amphiphilic Nanocomplexes Alleviates Deficits in Mouse Models of Alzheimer’s Disease. Nat Neurosci. 2019;22:524–528. doi: 10.1038/s41593-019-0352-0. [DOI] [PubMed] [Google Scholar]
- 376.He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP. Gamma-Secretase Activating Protein Is a Therapeutic Target for Alzheimer’s Disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wong E, Liao GP, Chang JC, Xu P, Li Y-M, Greengard P. GSAP Modulates γ-Secretase Specificity by Inducing Conformational Change in PS1. Proc Natl Acad Sci. 2019;116:6385–6390. doi: 10.1073/pnas.1820160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.György B, Lööv C, Zaborowski MP, Takeda S, Kleinstiver BP, Commins C, Kastanenka K, Mu D, Volak A, Giedraitis V. CRISPR/Cas9 Mediated Disruption of the Swedish APP Allele as a Therapeutic Approach for Early-Onset Alzheimer’s Disease. Mol Ther-Nucleic Acids. 2018;11:429–440. doi: 10.1016/j.omtn.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Nagata K, Takahashi M, Matsuba Y, Okuyama-Uchimura F, Sato K, Hashimoto S, Saito T, Saido TC. Generation of App Knock-in Mice Reveals Deletion Mutations Protective against Alzheimer’s Disease-like Pathology. Nat Commun. 2018;9:1–7. doi: 10.1038/s41467-018-04238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Schmid B, Prehn KR, Nimsanor N, Garcia BIA, Poulsen U, Jørring I, Rasmussen MA, Clausen C, Mau-Holzmann UA, Ramakrishna S. Generation of a Set of Isogenic, Gene-Edited IPSC Lines Homozygous for All Main APOE Variants and an APOE Knock-out Line. Stem Cell Res. 2019;34:101349. doi: 10.1016/j.scr.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 381.Wadhwani AR, Affaneh A, Van Gulden S, Kessler JA. Neuronal Apolipoprotein E4 Increases Cell Death and Phosphorylated Tau Release in Alzheimer Disease. Ann Neurol. 2019;85:726–739. doi: 10.1002/ana.25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Raikwar SP, Thangavel R, Dubova I, Selvakumar GP, Ahmed ME, Kempuraj D, Zaheer SA, Iyer SS, Zaheer A. Targeted Gene Editing of Glia Maturation Factor in Microglia: A Novel Alzheimer’s Disease Therapeutic Target. Mol Neurobiol. 2019;56:378–393. doi: 10.1007/s12035-018-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Soldner F, Stelzer Y, Shivalila CS, Abraham BJ, Latourelle JC, Barrasa MI, Goldmann J, Myers RH, Young RA, Jaenisch R. Parkinson-Associated Risk Variant in Distal Enhancer of α-Synuclein Modulates Target Gene Expression. Nature. 2016;533:95–99. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Qing X, Walter J, Jarazo J, Arias-Fuenzalida J, Hillje A-L, Schwamborn JC. CRISPR/Cas9 and PiggyBac-Mediated Footprint-Free LRRK2-G2019S Knock-in Reveals Neuronal Complexity Phenotypes and α-Synuclein Modulation in Dopaminergic Neurons. Stem Cell Res. 2017;24:44–50. doi: 10.1016/j.scr.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 385.Song C, Charli A, Luo J, Riaz Z, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. Mechanistic Interplay between Autophagy and Apoptotic Signaling in Endosulfan-Induced Dopaminergic Neurotoxicity: Relevance to the Adverse Outcome Pathway in Pesticide Neurotoxicity. Toxicol Sci. 2019;169:333–352. doi: 10.1093/toxsci/kfz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Gordon R, Neal ML, Luo J, Langley MR, Harischandra DS, Panicker N, Charli A, Jin H, Anantharam V, Woodruff TM. Prokineticin-2 Upregulation during Neuronal Injury Mediates a Compensatory Protective Response against Dopaminergic Neuronal Degeneration. Nat Commun. 2016;7:1–18. doi: 10.1038/ncomms12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Chen Y, Dolt KS, Kriek M, Baker T, Downey P, Drummond NJ, Canham MA, Natalwala A, Rosser S, Kunath T. Engineering Synucleinopathy-Resistant Human Dopaminergic Neurons by CRISPR-Mediated Deletion of the SNCA Gene. Eur J Neurosci. 2019;49:510–524. doi: 10.1111/ejn.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Kempuraj D, Dubova I, Saeed D, Zahoor H, Premkumar K, Zaheer S. CRISPR/Cas9 Editing of Glia Maturation Factor Regulates Mitochondrial Dynamics by Attenuation of the NRF2/HO-1 Dependent Ferritin Activation in Glial Cells. J Neuroimmune Pharmacol. 2019;14:537–550. doi: 10.1007/s11481-019-09833-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Nance MA. Genetics of Huntington Disease. Handb Clin Neurol. 2017;144:3–14. doi: 10.1016/B978-0-12-801893-4.00001-8. [DOI] [PubMed] [Google Scholar]
- 390.Wild EJ, Tabrizi SJ. Therapies Targeting DNA and RNA in Huntington’s Disease. Lancet Neurol. 2017;16:837–847. doi: 10.1016/S1474-4422(17)30280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Monteys AM, Ebanks SA, Keiser MS, Davidson BL. CRISPR/Cas9 Editing of the Mutant Huntingtin Allele in Vitro and in Vivo. Mol Ther. 2017;25:12–23. doi: 10.1016/j.ymthe.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Wu J, Ryskamp D, Birnbaumer L, Bezprozvanny I. Inhibition of TRPC1-Dependent Store-Operated Calcium Entry Improves Synaptic Stability and Motor Performance in a Mouse Model of Huntington’s Disease. J Huntingt Dis. 2018;7:35–50. doi: 10.3233/JHD-170266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Lee J, Park EH, Couture G, Harvey I, Garneau P, Pelletier J. An Upstream Open Reading Frame Impedes Translation of the Huntingtin Gene. Nucleic Acids Res. 2002;30:5110–5119. doi: 10.1093/nar/gkf664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Höijer I, Tsai Y-C, Clark TA, Kotturi P, Dahl N, Stattin E-L, Bondeson M-L, Feuk L, Gyllensten U, Ameur A. Detailed Analysis of HTT Repeat Elements in Human Blood Using Targeted Amplification-Free Long-Read Sequencing. Hum Mutat. 2018;39:1262–1272. doi: 10.1002/humu.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Mitchell AM, Nicolson SC, Warischalk JK, Jude Samulski R. AAV’s Anatomy: Roadmap for Optimizing Vectors for Translational Success. Curr. Gene Ther. 2010;10:319–340. doi: 10.2174/156652310793180706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Kay MA. State-of-the-Art Gene-Based Therapies: The Road Ahead. Nat Rev Genet. 2011;12:316–328. doi: 10.1038/nrg2971. [DOI] [PubMed] [Google Scholar]
- 397.Schaffer DV, Koerber JT, Lim K. Molecular Engineering of Viral Gene Delivery Vehicles. Annu Rev Biomed Eng. 2008;10:169–194. doi: 10.1146/annurev.bioeng.10.061807.160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Cucchiarini M. Human Gene Therapy: Novel Approaches to Improve the Current Gene Delivery Systems. Discov Med. 2016;21:495–506. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are now available.