Abstract
The purpose of this study was to develop and validate a robust and sensitive LC–MS/MS method for simultaneous determinations of various tyrosine kinase inhibitors (TKIs) in biological samples and to apply the method to their pharmacokinetic studies. Processed samples were injected into the UHPLC system coupled to an ESI-triple quadrupole mass spectrometer. The compounds were separated on an AcQuity UHPLC BEH C18 column (50 mm × 2.1 mm ID, 1.7 μm) using a gradient elution of acetonitrile/0.1% formic acid in water. The mass analysis was performed in an API 3200 Qtrap mass spectrometer via selective reaction monitoring operated under a positive scanning mode. The method was validated over a linear range of 3.13–800 nM for erlotinib; 6.25–1600 nM for sunitinib, pazopanib, and axitinib; and 12.5–3200 nM for sorafenib, dasatinib, lapatinib, and nilotinib, respectively. The intra-day and inter-day precision were <16.7% for quality control samples of the analytes at the low concentration level and <13.7% for all other concentrations. The accuracy (bias) for all analytes at three different concentration levels ranged from −12.2% to 15.0%. The recovery, matrix effect, and stability were all in the range of acceptance. Only 10 μl of blood were needed, demonstrating the method’s high sensitivity. The presented method was shown to be suitable for the analysis of serial blood samples collected from each mouse in a pharmacokinetic study, after the oral administration of 11 TKIs (each at 1 mg/kg) as a mixture.
Keywords: Tyrosine kinase inhibitors, UHPLC–MS/MS, Mice pharmacokinetics
1. Introduction
Tyrosine kinase inhibitors (TKIs) have revolutionized the treatment of many cancers as a targeted therapy in the last two decades. Since the first TKI imatinib was approved in 2001 by the FDA, a total of ~30 TKIs have been approved as of 2016 [1]. TKIs are small molecules that are orally administered to target specific tyrosine kinases. Tyrosine kinases are important enzymes that regulate cellular signal transduction, as well as cellular proliferation, differentiation, migration and apoptosis. Deregulation of tyrosine kinase function (e.g., via mutation) has been implicated in the development of many cancers. TKIs mediate the anti-tumor effects by interfering in the specific molecule pathways involved in tumor cell growth [2,3].
Despite the great benefits gained from TKI therapy, increasing cases of treatment failure due to drug resistance and toxicity have been reported [4,5]. There are a variety of underlying mechanisms leading to acquired resistance, including point mutations within the kinase domain, bypass of signaling pathways, and the overexpression of ABC (ATP-binding cassette) transporters [6–8]. One of the most promising approaches in overcoming the resistance utilizes different combinations of TKIs to target additional tyrosine kinases beyond the primary target, or these combinations may reverse the pathway responsible for transporter-mediated resistance [8–11]. Compared to conventional chemotherapy, TKIs have well-defined molecular targets and different toxicity profiles. The most common side effects of TKIs are rashes, diarrhea, hypertension, bleeding, and hepatotoxicity [5,12,13]. Hence, more effective therapies via combinational approach are warranted in order to improve the therapeutic outcomes. In fact, several preclinical and clinical trials of combining different TKIs are actively ongoing [11,14–16]. Moreover, TKIs show high interpatient pharmacokinetics variability, possibly due to food intake, co-administered drug, disease or other factors. This variation in drug levels would lead to drug toxicity or sub-therapeutic efficacy. Several reports have suggested that plasma concentrations are more predictive than administered dose in predicting treatment response [17–19]. Therefore, in order to explore potential TKIs combinations, investigate the drug-drug interactions (DDI) of different TKIs, and monitor the targeted drug exposure after combination therapy, it is essential to develop a robust and sensitive bioanalytical assay to quantify commonly used TKIs simultaneously.
Several analytical methods using UHPLC–MS/MS for quantification of various TKIs have been published so far. Most of the assays were developed for the determination of a single TKI or its metabolites. A few methods were able to quantify more than six TKIs simultaneously. Bouchet et al. reported an assay quantifying 9 TKIs simultaneously using 96-well solid-phase extraction [20]. Andriamanana et al. and Huynh et al. described the methods for the simultaneous analysis of 9 TKIs and 14 TKIs in human plasma, respectively [21,22]. However, all these methods required 50–300 μl plasma for the sample preparation, which is difficult to achieve in small animal models, especially mice. Therefore, to fulfill this gap, we developed and validated a simple and sensitive UHPLC–MS/MS method for the simultaneous quantification of 8 commercial TKIs in rodent blood, erlotinib, sorafenib, sunitinib, dasatinib, lapatinib, nilotinib, pazopanib, and axitinib. All procedures followed the FDA guidelines for bioanalytical method validation, because of need to perform the preclinical pharmacokinetic studies of various TKIs when used in combination [23].
2. Experimental
2.1. Chemicals and reagents
Imatinib, gefitinib, erlotinib hydrochloride, sorafenib tosylate, sunitinib malate, dasatinib, lapatinib, nilotinib, pazopanib hydrochloride, axitinib, and afatinib were purchased from LC laboratories (Woburn, MA, USA). Testosterone was purchased from Sigma–Aldrich (St. Louis, MO, USA). Acetonitrile, methanol, and water (LC–MS grade) were purchased from EMD (Gibbstown, NJ, USA). Formic acid was purchased from Fisher Chemical (Pittsburgh, PA, USA). DMSO was purchased from Corning (Manassas, VA, USA). All other chemicals were used as received.
2.2. Instruments and conditions
2.2.1. UHPLC
The ultra high-performance liquid chromatography (UHPLC) conditions for the separation of 8 TKIs and testosterone (IS) were the following: system, Waters Acquity™ with diode array detector (DAD); column, Acquity UHPLC BEH C18 column (50 mm × 2.1 mm I.D., 1.7 μm, Waters, Milford, MA. USA); mobile phase A, 0.1% formic acid in water; mobile phase B, 100% acetonitrile; gradient: 0–0.5 min, 10% B, 0.5–1.5 min, 10–30% B, 1.5–3.5 min, 30–70% B, 3.5–3.8 min, 70–95% B, 3.8–5.5 min, 95% B, 5.5–6.0 min, 95–10% B, 6.0–6.5 min, 10% B; flow rate, 0.45 ml/min; column temperature, 45°C; sample temperature, 10°C; and injection volume, 10 μl.
2.2.2. MS
The MS analysis was performed on an API 3200 Qtrap triple quadrupole mass spectrometer (AB Sciex, Foster City, CA, USA) equipped with a TurboIon Spray™ source. The detection of the agents was performed by using selective reaction monitoring (SRM) scan type in the positive ion mode. The instrument dependent parameters were set as follows: ionspray voltage, 5.5 kV; ion source temperature, 550°C; nebulizer gas (gas 1), nitrogen, 20 psi; turbo gas (gas 2), nitrogen, 40 psi; curtain gas, nitrogen, 30 psi. The selected m/z transitions and compound dependent parameters were listed in Table 1 [24–27].
Table 1.
Compound-dependent parameters of eight tyrosine kinase inhibitors with testosterone as internal standard (IS) in UHPLC–MS/MS analysis.
| Analyte | Precursor ion (m/z) | Product ion (m/z) | Dwell time (ms) | DP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|
|
| ||||||
| Erlotinib | 394.3 | 278.3 | 100 | 60 | 40 | 3.6 |
| Sorafenib | 465.1 | 270.2 | 100 | 55 | 30 | 4.0 |
| Sunitinib | 399.4 | 283.1 | 100 | 50 | 38 | 3.5 |
| Dasatinib | 488.3 | 401.2 | 100 | 67 | 40 | 5.0 |
| Lapatinib | 581.2 | 365.2 | 100 | 87 | 48 | 5.0 |
| Nilotinib | 530.3 | 289.2 | 100 | 84 | 39 | 3.0 |
| Pazopanib | 438.3 | 357.3 | 100 | 88 | 38 | 4.0 |
| Axitinib | 387.0 | 356.2 | 100 | 57 | 26 | 5.0 |
| Testosterone (IS) | 288.9 | 97.0 | 100 | 57 | 39 | 4.0 |
DP: declustering potential; CE: collision energy; CXP: collision cell exit potential.
2.3. Preparation of calibration standards and quality control samples
The stock solutions for each analyte, erlotinib, sorafenib, sunitinib, dasatinib, lapatinib, nilotinib, pazopanib, and axitinib, were prepared in DMSO at a concentration of 4 mM. The stock solutions were stored in amber glass containers at −20°C. To prepare standard curve samples in blood, the stock solutions of different analytes were mixed and further diluted with acetonitrile to obtain the working solutions from 31.3 to 8000 nM for erlotinib; 62.5–16000 nM for sunitinib, pazopanib, and axitinib; and 125–32000 nM for sorafenib, dasatinib, lapatinib, and nilotinib. Subsequently, the working solutions (10 μl) were spiked into 10 μl blank rodent blood, and 100 μl internal standard (2 μM testosterone in acetonitrile) was added for protein precipitation. The mixture was vortexed vigorously for 1 min and centrifuged at 15,000 rpm for 15 min. Then a volume of 100 μl supernatant was transferred to a new tube and evaporated to dryness under a stream of air. The residue was reconstituted in 100 μl of 50% acetonitrile. After centrifuge at 15,000 rpm for 15 min, 10 μl of the supernatant was injected for analysis. The final concentrations of these agents were 3.125, 6.25, 12.5, 25, 50, 100, 200, 400, 800 nM for erlotinib; 6.25, 12.5, 25, 50, 100, 200, 400, 800, 1600 nM for sunitinib, pazopanib, and axitinib; and 12.5, 25, 50, 100, 200, 400, 800, 1600, 3200 nM for sorafenib, dasatinib, lapatinib, and nilotinib. The quality control (QC) samples at three different concentrations were prepared in the same way as calibration standards.
2.4. Method validation
2.4.1. Linearity and lower limit of quantification
Calibration curves were prepared as described in 2.3. The linearity of each calibration curve was determined by plotting the peak area ratio of each analyte to IS versus the concentrations of the analyte. A least-squares linear regression method (1/x2 weight) was fitted to determine the slope, intercept and correlation coefficient of linear regression equation. The lower limit of quantification (LLOQ) is the lowest concentration which could be determined accurately and precisely with a signal-to-noise ratio (S/N) of at least 5:1.
2.4.2. Selectivity
The selectivity was assessed by comparing the chromatograms of eight different batches of blank mice blood with the corresponding spiked LLOQ blood samples. As a multi-analyte assay, cross-analyte and IS interference was evaluated by spiking blank blood samples with each analyte (the 8 studied TKIs in the assay, imatinib, gefitinib, and afatinib) at upper limit of quantification (ULOQ) level and IS at working concentration separately. The method is considered selective if the response is less than 20% of analyte at LLOQ level and less than 5% for IS at the working concentration.
2.4.3. Precision and accuracy
The intra-day and inter-day precision and accuracy were determined by analysis of QC samples at three different concentrations (low, medium, and high) on the same day or three different days. The coefficient of variation (CV, %) was taken as a measure of precision, and the percentage of deviation between the measured concentration and the nominal value was considered a measure of accuracy.
2.4.4. Recovery and matrix effect
The extraction recovery of the 8 TKIs was determined by comparing the peak areas obtained from samples where blank blood was spiked with the analytes before the extraction against samples where the analytes were spiked into these post-extracted blood samples. The matrix effect was determined by comparing the response obtained from blank blood extracts spiked with analytes to those aqueous standards dried and reconstituted. Both recovery and matrix effect were examined at low, medium, and high concentration QC levels for each drug.
2.4.5. Stability
The stabilities of these compounds were evaluated by analyzing three replicates of QC samples at low and high concentrations following storage at room temperature for 8 h, at −80°C for 7 days, and after three freeze-thaw cycles (from −80°C to 25°C). The postpreparative stability was determined by keeping the QC samples in the autosampler at 10°C for 12 h. The stock solutions of each analyte were also evaluated for stability at −20°C in dark for ~2 months.
2.5. Application of the method for mice pharmacokinetics study
2.5.1. Animals
The animal protocols used in this study were approved by the University of Houston’s Institutional Animal Care and Use Committee. Male FVB wild-type mice (25–30 g, 10–12 weeks old) were from Harlan Laboratory (Indianapolis, IN) and kept in an environmentally controlled room (temperature: 25 ± 2°C, humidity: 50 ± 5%, 12 h dark-light cycle) for at least 1 week before the experiments.
2.5.2. Experimental design
The mice were fasted overnight with free access to water before the day of the experiment. A mixture of 11 TKIs (imatinib, gefitinib, afatinib, and the 8 studied drugs in the assay) was suspended in oral suspension vehicle with a final concentration of 0.06 mg/ml for each and administered to mice by oral gavage at a dose of 1 mg/kg. Blood samples (about 15 μl) were collected in heparinized tubes from the tip of the mouse tail at 15, 30, 45, 60, 120, 240, 360, 480, and 1440 min. The blood samples were stored at −80°C until analysis.
2.5.3. Sample preparation
The blood sample (10 μl) was spiked with 10 μl of acetonitrile and 100 μl of IS. The following steps were in the same way as described in Section 2.3, except that the final residue was reconstituted in 50 μl of 50% acetonitrile, instead of 100 μl, in order to increase the response of each analyte.
2.5.4. Pharmacokinetic parameters calculation
The pharmacokinetic parameters were calculated by the non-compartmental model using WinNonlin 3.3 (Pharsight, Mountain View, CA).
3. Results and discussion
3.1. Method development
The method was developed by optimizing both UHPLC and MS conditions to obtain the optimal peak shapes, chromatographic separation, and sensitivity. Ammonium acetate (2.5 mM, pH 6.5), and 0.1–0.2% formic acid in water were used as mobile phase A. Methanol, acetonitrile, and 0.1–0.2% formic acid in acetonitrile were used as mobile phase B. The mobile phases were finally set as 0.1% formic acid in water (mobile phase A) and acetonitrile (mobile phase B) according to the peak separation, shape and intensity. The analysis was 6.5 min per run. All the TKIs and IS were eluted within 4 min, followed by a 1.7 min rinsing step with 95% acetonitrile and a 0.5 min conditioning step with 10% acetonitrile. No carryover was detected in the blank blood sample after injecting the calibration standard with the highest concentration.
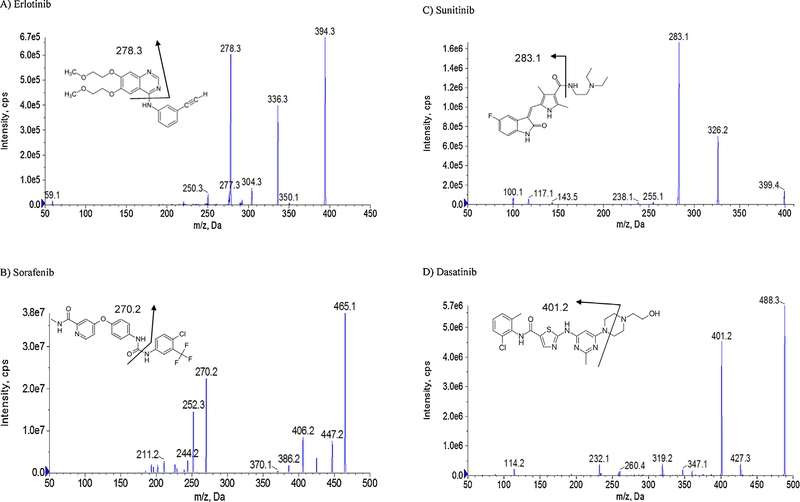
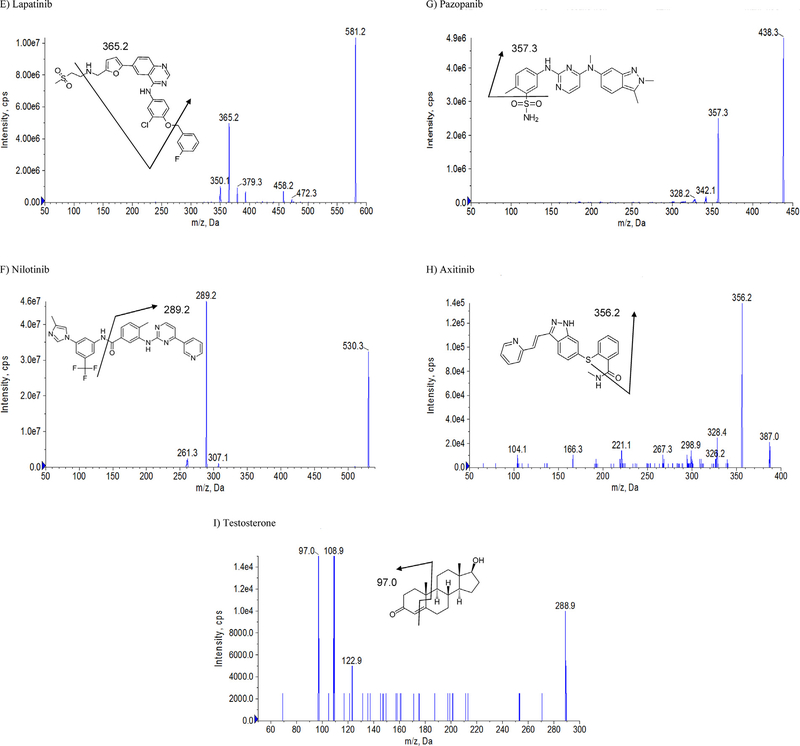
For the MS conditions, positive mode was selected for all agents. Selective reaction monitoring (SRM) scan type was used to improve the specificity in this analysis. The product ion with the highest abundance was selected as the transition for each analyte. The product ion spectra and the proposed fragmentation pathways for the eight TKIs and IS are shown in Fig. 1 [24–27]. To improve the sensitivity, the instrument dependent parameters and the compound dependent parameters were optimized by tuning these analytes separately. Testosterone was selected as IS because it possesses the chromatography behavior that is similar to the analytes of interest. The optimized MS/MS transitions and the compound dependent parameters of all the analytes and IS are summarized in Table 1 [24–27]. A typical chromatogram of a standard sample containing all TKIs and IS is shown in Fig. 2. Sunitinib was present with two peaks at the same MS/MS transition (m/z 399.4 → 283.1) because of an E/Z isomerization reaction as reported before. It has been demonstrated that the transition of E/Z configurations with an equal MS response is induced by light exposure and is reversible [28,29]. Therefore, the sum of both sunitinib isomers was used for quantification.
Fig. 1.
The MS/MS chromatograms and proposed fragmentation pathways for the eight tyrosine kinase inhibitors (TKIs) and testosterone (IS).
Fig. 2.
A representative SRM chromatogram of a calibration standard spiked in blank blood containing 1) sunitinib, 2) pazopanib, 3) axitinib, 4) dasatinib, 5) erlotinib, 6) nilotinib, 7) lapatinib, 8) sorafenib, and 9) testosterone (IS).
3.2. Method validation
The method was validated in agreement with the Food and Drug Administration (FDA) guidance for bioanalytical method validation [23].
3.2.1. Linearity and lower limit of quantification
The standard curve was linear from 3.13 to 800 nM for erlotinib; 6.25–1600 nM for sunitinib, pazopanib and axitinib; and 12.5–3200 nM for sorafenib, dasatinib, lapatinib, and nilotinib. The correlation coefficients (r2) were at least 0.99 for all calibration curves. The accuracies were within 85% − 115% for the blood calibration standards at all concentration levels. The lower limit of quantification (LLOQ) was 3.13 nM for erlotinib; 6.25 nM for sunitinib, pazopanib, and axitinib; and 12.5 nM for sorafenib, dasatinib, lapatinib, and nilotinib.
3.2.2. Selectivity
Selectivity is the ability of an analytical method to differentiate and quantify the analyte in the presence of other components in the sample [23]. No peaks from endogenous matrix components were observed at the retention time of all the analytes and IS in the blank blood from the eight mice. In addition, as the method was applied to quantify more than one analyte, it was also important to check the potential interference from different compounds that might be present in the real samples. Therefore, in addition to the 8 studied TKIs in the present assay, another three commonly used TKIs (imatinib, gefitinib, and afatinib) in patients were also added to blank blood separately to assess the cross-interference. The response detected at the retention time for each TKI was <20% of that at LLOQ and <5% for IS at working concentration, which was within the acceptance criteria.
3.2.3. Precision and accuracy
The intra-day and inter-day precision and accuracy were determined by analyzing QC samples at low, medium, and high concentration levels of the calibration range with five replicates. The assay performance is summarized in Table 2. The intra-day precision was <14.3% for all QC samples at three different concentration levels. The inter-day precision of the analytes was <9.6% at high and medium concentration levels and <16.7 for low concentrations. The intra-day and inter-day deviation (bias) from the nominal concentrations was within ±15% for all analytes at all concentration levels. The results showed that the precision and accuracy fell into the acceptance range, suggesting that the method was reproducible for the quantification of the investigated TKIs.
Table 2.
Intra-day and inter-day accuracy and precision of eight TKIs from the assay samples.
| Intra-day |
Inter-day |
||||
|---|---|---|---|---|---|
| Analyte | Nominal Concentration (nM) | Accuracy (bias, %) | Precision (CV, %) | Accuracy (bias, %) | Precision (CV, %) |
|
| |||||
| Erlotinib | 400 | −4.2 | 3.1 | −7.5 | 4.2 |
| 50 | 5.2 | 7.3 | 9.6 | 6.5 | |
| 6.25 | 9.2 | 10.1 | −0.6 | 14.9 | |
| Sorafenib | 1600 | 0.8 | 3.0 | 1.4 | 2.6 |
| 200 | 9.3 | 5.4 | 13.7 | 4.8 | |
| 25 | 15.0 | 6.5 | 12.3 | 7.5 | |
| Sunitinib | 800 | −12.0 | 2.5 | −12.2 | 2.7 |
| 100 | 7.8 | 8.8 | 11.3 | 5.6 | |
| 12.5 | 14.6 | 4.7 | 13.3 | 5.6 | |
| Dasatinib | 1600 | −1.5 | 3.7 | −3.2 | 3.4 |
| 200 | 5.8 | 4.0 | 8.3 | 4.4 | |
| 25 | 8.7 | 11.7 | 1.1 | 12.7 | |
| Lapatinib | 1600 | −11.6 | 7.2 | −10.1 | 6.1 |
| 200 | −0.2 | 7.0 | 0.6 | 9.6 | |
| 25 | 0.4 | 14.3 | 0.8 | 10.4 | |
| Nilotinib | 1600 | −6.5 | 6.2 | −10.7 | 6.2 |
| 200 | 6.5 | 6.5 | 6.7 | 6.1 | |
| 25 | 6.6 | 7.5 | −1.8 | 13.1 | |
| Pazopanib | 800 | 1.5 | 4.7 | −9.4 | 9.5 |
| 100 | 0.9 | 5.6 | −6.1 | 7.4 | |
| 12.5 | 8.6 | 13.1 | −7.1 | 16.7 | |
| Axitinib | 800 | −5.1 | 3.8 | −8.8 | 5.4 |
| 100 | 0.9 | 5.3 | 0.0 | 3.8 | |
| 12.5 | 4.5 | 7.8 | −0.9 | 11.2 | |
3.2.4. Extraction recovery and matrix effect
The extraction recovery and matrix effect were determined using three replicates of QC samples at three concentration levels as listed in Table 3. Acetonitrile was used to extract the 8 TKIs in blood samples. The recoveries were not less than 85% for all analytes at low, medium, and high concentration levels, indicating that the analyte was recovered during the sample preparation process.
Table 3.
Extraction recovery and matrix effect of eight TKIs.
| Analyte | Nominal Concentration (nM) | Extraction Recovery (%) (n=3) | Matrix Effect (%) (n=3) |
|---|---|---|---|
|
| |||
| Erlotinib | 400 | 87.6 | 96.6 |
| 50 | 100.3 | 100.7 | |
| 6.25 | 91.6 | 110.7 | |
| Sorafenib | 1600 | 106.5 | 110.1 |
| 200 | 106.8 | 111.0 | |
| 25 | 105.2 | 118.9 | |
| Sunitinib | 800 | 113.8 | 103.4 |
| 100 | 104.6 | 97.4 | |
| 12.5 | 110.1 | 82.3 | |
| Dasatinib | 1600 | 85.0 | 104.4 |
| 200 | 96.2 | 102.1 | |
| 25 | 102.2 | 93.6 | |
| Lapatinib | 1600 | 97.4 | 110.3 |
| 200 | 100.1 | 105.1 | |
| 25 | 108.3 | 116.8 | |
| Nilotinib | 1600 | 96.3 | 97.4 |
| 200 | 108.3 | 114.1 | |
| 25 | 116.0 | 113.7 | |
| Pazopanib | 800 | 101.1 | 85.1 |
| 100 | 100.7 | 85.1 | |
| 12.5 | 93.8 | 82.9 | |
| Axitinib | 800 | 102.0 | 92.1 |
| 100 | 93.2 | 88.5 | |
| 12.5 | 88.2 | 90.8 | |
As for the matrix effects on the LC–MS analysis, the results showed that the relative peak areas of the analytes after spiking evaporated blood samples at three concentration levels were comparable to those similarly prepared aqueous standard solutions, which suggested that the method was not adversely affected by matrix effects in the quantification of the analytes in blood.
3.2.5. Stability
Short-term (25°C for 8 h), long-term (−80°C for 7 days), three freeze-thaw cycles, and post-preparation stabilities were determined for all analytes in triplicate at each of the low and high concentrations. No significant degradation was observed under these storage conditions. The concentrations of all analytes in the stability test samples fell within 85%–115% of the concentrations in the fresh prepared controls. The results of the stock solution stability experiments showed that all the analytes were stable when stored at −20°C for ~2 months. The percentage of deviation for the measured concentrations between post-storage and the freshly prepared stock solutions was less than 10% for each analyte.
3.3. Application in pharmacokinetic study in mice
The applicability of the validated method was demonstrated by studying the pharmacokinetic behaviors of 11 TKIs (at 1 mg/kg each) as a mixture in FVB wild-type mice (n = 4) after an oral administration. In addition to the eight studied drugs in the present method, another three TKIs, imatinib, gefitinib, and afatinib were added to determine if the presence of these three agents would interfere with the determination of the eight investigated TKIs, as well as the feasibility of the method to be extended to analyze more TKIs.
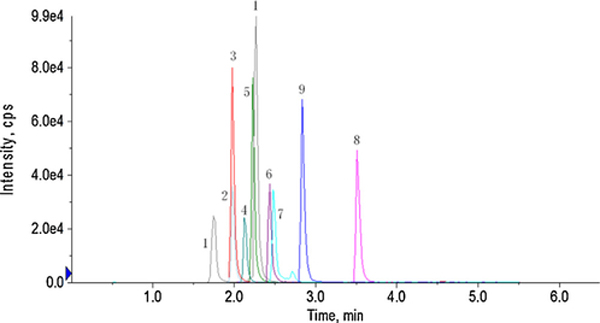
The results showed that at this dose, only pazopanib and sorafenib produced complete 24 h pharmacokinetic profiles. The mean blood concentration-time profiles of pazopanib and sorafenib are shown in Fig. 3. The estimated pharmacokinetic parameters are listed in Table 4. It revealed that pazopanib reached a maximum concentration (Cmax) of 717.0 ± 205.5 ng/ml at approximately 97 min. For sorafenib, the Cmax was 154.3 ± 30.4 ng/ml, and Tmax was 60 min. The AUC (area under the curve) values reflected that the systemic exposure to pazopanib (248812.5 ± 65971.3 min ng/ml) was 7.5 times higher than that of sorafenib (33489.4 ± 6766.4 min ng/ml) in mice.
Fig. 3.
Blood concentration of sorafenib and pazopanib after oral administration of 1 mg/kg as a mixture of 11 TKIs in FVB wild-type mice. Each point is the average of four determinations and the error bars are standard deviations of the mean.
Table 4.
Pharmacokinetic parameters of sorafenib and pazopanib after oral administration of 1 mg/kg as a mixture of 11 TKIs in mice (n = 4).
| Analyte | Cmax (ng/ml) | Tmax (min) | AUC0-t (min ng/ml) |
|---|---|---|---|
|
| |||
| Sorafenib | 154.3 ± 30.4 | 60.0 ± 40.6 | 33489.4 ± 6766.4 |
| Pazopanib | 717.0 ± 205.5 | 97.5 ± 66.5 | 248812.5 ± 65971.3 |
The other compounds could only be detected at certain time point(s) due to low dose as summarized in Table 5. Erlotinib reached the Cmax 147.1 ± 62.2 ng/ml in 15 min, suggesting that erlotinib was absorbed rapidly after administration. No interference was observed for the determination of the drugs studied in the assay at the presence of imatinib, gefitinib, and afatinib as described in Section 3.2.2. In addition, both imatinib and gefitinib could also be detected and possibly measured by simply adjusting their compound-dependent mass parameters to the present assay. Afatinib was not detected at this dose.
Table 5.
The concentration (mean ± SD, n = 4, ng/ml) of those compounds only detected at certain time points in the PK study.
| Analyte | Time (min) |
|||||
|---|---|---|---|---|---|---|
| 15 | 30 | 45 | 60 | 120 | 240 | |
|
| ||||||
| Erlotinib | 147.1 ± 62.2 | 123.7 ± 45.0 | 119.5 ± 97.6 | 87.6 ± 87.6 | 23.0 ± 37.7a | 14.4 ± 24.9a |
| Sunitinib | 9.4 ± 12.1a | 18.4 ± 10.6a | 22.8 ± 18.9a | 12.7 ± 20.0a | 14.7 ± 15.8a | ND |
| Dasatinib | 9.1 ± 18.2a | 21.5 ± 19.2a | 34.3 ± 41.6a | 24.8 ± 49.7a | 18.4 ± 23.1a | 10.1 ± 20.2a |
| Lapatinib | 8.8 ± 12.7a | 16.3 ± 28.2a | 21.4 ± 25.6a | 21.2 ± 25.7a | 20.1 ± 28.2a | 18.1 ± 25.4a |
| Nilotinib | 62.1 ± 32.8 | 59.1 ± 31.4 | 69.0 ± 37.2 | 68.8 ± 26.8 | 67.3 ± 64.3 | 34.6 ± 33.1a |
| Axitinib | 51.7 ± 40.0 | 42.7 ± 19.3 | 46.1 ± 39.6 | 34.2 ± 37.3 | 11.1 ± 16.4a | 4.8 ± 26.8a |
| Imatinib | 53.1 ± 20.1 | 55.7 ± 28.6 | 68.8 ± 63.8 | 48.1 ± 56.9 | 19.9 ± 19.0a | 11.1 ± 19.2a |
| Gefitinib | 24.7 ± 22.0 | 32.4 ± 28.1a | 36.7 ± 41.5a | 33.2 ± 42.7a | 21.8 ± 25.5a | 32.7 ± 26.3a |
ND, not detected.
concentrations for some mouse blood samples fell below the quantification limit, for which zero was used to replace the actual numbers.
Although we successfully applied the validated method for the simultaneous measurement of the selected TKIs, we were not able to fully describe their PK profiles because the concentrations at some sampling points were not measurable (below LLOQ). Because of the distinct pharmacokinetic characteristics and complex drug-drug interactions among these compounds, PK studies with higher and yet reasonable doses for different agents are needed in order to determine their PK parameters and profiles [30–33].
4. Conclusion
A rapid, specific and sensitive UHPLC–MS/MS method was developed and validated for the simultaneous determination of 8 tyrosine kinase inhibitors in rodent blood. The main advantages of this method are: (1) a small volume of blood sample (10 μl) is needed; (2) sample preparation procedures are simple and fast; (3) rapid analysis (6.5 min); (4) good recovery and minor matrix effect; and (5) the IS (testosterone) is easy to access for lab experiments. The method was successfully applied to the pharmacokinetic study in mice. In addition, even though eight TKIs were studied as representatives for this class of drugs in the present method, this assay is expected to be applied to the analysis of other TKIs as these drugs possess similar chemical properties. Furthermore, this method is also valuable for therapeutic drug monitoring in patients as well as the investigation of drug-drug interactions of different TKIs in clinical trials. It should allow a higher sensitivity than that reported here because a larger volume of blood sample is usually available in human, which may be used to concentrate the compounds of interest before analysis.
Acknowledgement
Work supported by NIH GM070737 to MH and NIH CA195504 to XW.
References
- [1].Wu P, Nielsen TE, Clausen MH, Small-molecule kinase inhibitors: an analysis of FDA-approved drugs, Drug Discov. Today 21 (1) (2016) 5–10. [DOI] [PubMed] [Google Scholar]
- [2].Paul MK, Mukhopadyay AK, Tyrosine kinase −role and significance in cancer, Int. J. Med. Sci. 1 (2) (2004) 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Levitzki A, Tyrosine kinase inhibitors: views of selectivity, sensitivity, and clinical performance, Annu. Rev. Pharmacol. Toxicol. 53 (2013) 161–185. [DOI] [PubMed] [Google Scholar]
- [4].Apperley JF, Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia, Lancet Oncol. 8 (11) (2007) 1018–1029. [DOI] [PubMed] [Google Scholar]
- [5].Sodergren SC, White A, Efficace F, Sprangers M, Fitzsimmons D, Bottomley A, Johnson CD, Systematic review of the side effects associated with tyrosine kinase inhibitors used in the treatment of gastrointestinal stromal tumours on behalf of the EORTC Quality of Life Group, Crit. Rev. Oncol. Hematol. 91 (1) (2014) 35–46. [DOI] [PubMed] [Google Scholar]
- [6].Alexander PB, Wang XF, Resistance to receptor tyrosine kinase inhibition in cancer: molecular mechanisms and therapeutic strategies, Front. Med. 9 (2) (2015) 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen YF, Fu LW, Mechanisms of acquired resistance to tyrosine kinase inhibitors, Acta Pharm. Sin. B 1 (4) (2011) 197–207. [Google Scholar]
- [8].Ravaud A, Gross-Goupil M, Overcoming resistance to tyrosine kinase inhibitors in renal cell carcinoma, Cancer Treat. Rev. 38 (8) (2012) 996–1003. [DOI] [PubMed] [Google Scholar]
- [9].Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J, Zweibel J, Collins J, Doroshow JH, Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements, Nat. Rev. Drug Discov. 9 (11) (2010) 843–856. [DOI] [PubMed] [Google Scholar]
- [10].D’Cunha R, Bae S, Murry DJ, An G, TKI combination therapy: strategy to enhance dasatinib uptake by inhibiting Pgp- and BCRP-mediated efflux, Biopharm. Drug Dispos. 37 (7) (2016) 397–408. [DOI] [PubMed] [Google Scholar]
- [11].Nehoff H, Parayath NN, McConnell MJ, Taurin S, Greish K, A combination of tyrosine kinase inhibitors, crizotinib and dasatinib for the treatment of glioblastoma multiforme, Oncotarget 6 (5) (2015) 37948–37964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shah DR, Shah RR, Morganroth J, Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy, Drug Saf. 36 (6) (2013) 413–426. [DOI] [PubMed] [Google Scholar]
- [13].Terada T, Noda S, Inui K, Management of dose variability and side effects for individualized cancer pharmacotherapy with tyrosine kinase inhibitors, Pharmacol. Ther. 152 (2015) 125–134. [DOI] [PubMed] [Google Scholar]
- [14].Scagliotti GV, Krzakowski M, Szczesna A, Strausz J, Makhson A, Reck M, Wierzbicki RF, Albert I, Thomas M, Miziara JE, Papai ZS, Karaseva N, Thongprasert S, Portulas ED, von Pawel J, Zhang K, Selaru P, Tye L, Chao RC, Govindan R, Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial, J. Clin. Oncol. 30 (17) (2012) 2070–2078. [DOI] [PubMed] [Google Scholar]
- [15].Gold KA, Lee JJ, Harun N, Tang X, Price J, Kawedia JD, Tran HT, Erasmus JJ, Blumenschein GR, William WN, Wistuba II, Johnson FM, A phase I/II study combining erlotinib and dasatinib for non-small cell lung cancer, Oncologist 19 (10) (2014) 1040–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Naud JS, Ghani K, de Campos-Lima PO, Caruso M, Nilotinib and imatinib inhibit cytarabine cellular uptake: implications for combination therapy, Leuk. Res. 36 (10) (2012) 1311–1314. [DOI] [PubMed] [Google Scholar]
- [17].Lankheet NAG, Knapen LM, Schellens JH, Beijnen JH, Steeghs N, Huitema ADR, Plasma concentrations of tyrosine kinase inhibitors imatinib, erlotinib, and sunitinib in routine clinical outpatient cancer care, Ther. Drug Monit. 36 (3) (2014) 326–334. [DOI] [PubMed] [Google Scholar]
- [18].Frankel C, Palmieri FM, Lapatinib side-effect management, Clin. J. Oncol. Nurs. 14 (2) (2010) 223–233. [DOI] [PubMed] [Google Scholar]
- [19].Yu H, Steeghs N, Nijenhuis CM, Schellens JH, Beijnen JH, Huitema AD, Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets, Clin. Pharmacokinet. 53 (4) (2014) 305–325. [DOI] [PubMed] [Google Scholar]
- [20].Bouchet S, Chauzit E, Ducint D, Castaing N, Canal-Raffin M, Moore N, Titier K, Molimard M, Simultaneous determination of nine tyrosine kinase inhibitors by 96-well solid-phase extraction and ultra performance LC/MS-MS, Clin. Chim. Acta. 412 (11–12) (2011) 1060–1067. [DOI] [PubMed] [Google Scholar]
- [21].Andriamanana I, Gana I, Duretz B, Hulin A, Simultaneous analysis of anticancer agents bortezomib imatinib, nilotinib, dasatinib, erlotinib, lapatinib, sorafenib, sunitinib and vandetanib in human plasma using LC/MS/MS, J. Chromatogr. B 926 (2013) 83–91. [DOI] [PubMed] [Google Scholar]
- [22].Huynh HH, Pressiat C, Sauvageon H, Madelaine I, Maslanka P, Lebbé C, Thieblemont C, Goldwirt L, Moura S, Development and validation of a simultaneous quantification method of 14 tyrosine kinase inhibitors in human plasma using LC-MS/MS, Ther. Drug Monit. 39 (2017) 43–54. [DOI] [PubMed] [Google Scholar]
- [23].Food and Drug Administration, Guidance for Industry: Bioanalytical Method Validation, 2013. [Google Scholar]
- [24].Lankheet NA, Hillebrand MJ, Rosing H, Schellens JH, Beijnen JH, Huitema AD, Method development and validation for the quantification of dasatinib, erlotinib, gefitinib, imatinib, lapatinib, nilotinib, sorafenib and sunitinib in human plasma by liquid chromatography coupled with tandem mass spectrometry, Biomed. Chromatogr. 27 (4) (2013) 466–476. [DOI] [PubMed] [Google Scholar]
- [25].van Erp NP, de Wit D, Guchelaar HJ, Gelderblom H, Hessing TJ, Hartigh J, A validated assay for the simultaneous quantification of six tyrosine kinase inhibitors and two active metabolites in human serum using liquid chromatography coupled with tandem mass spectrometry, J. Chromatogr. B 937 (2013) 33–43. [DOI] [PubMed] [Google Scholar]
- [26].Smith BJ, Pithavala Y, Bu HZ, Kang P, Hee B, Deese AJ, Pool WF, Klamerus KJ, Wu EY, Dalvie DK, Pharmacokinetics, metabolism, and excretion of [14C]axitinib, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in humans, Drug Metab. Dispos. 42 (5) (2014) 918–931. [DOI] [PubMed] [Google Scholar]
- [27].Thevis M, Beuck S, Höppner S, Thomas A, Held J, Schäfer M, Oomens J, Schänzer W, Structure elucidation of the diagnostic product ion at mz 97 derived from androst-4-en-3-one-based steroids by ESI-CID and IRMPD spectroscopy, J. Am. Soc. Mass Spectrom. 23 (3) (2012) 537–546. [DOI] [PubMed] [Google Scholar]
- [28].de Bruijn P, Sleijfer S, Lam MH, Mathijssen RH, Wiemer EA, Loos WJ, Bioanalytical method for the quantification of sunitinib and its n-desethyl metabolite SU12662 in human plasma by ultra performance liquid chromatography/tandem triple-quadrupole mass spectrometry, J. Pharm. Biomed. Anal. 51 (4) (2010) 934–941. [DOI] [PubMed] [Google Scholar]
- [29].Zhao Y, Sukbuntherng J, Antonian L, Simultaneous determination of Z-SU5416 and its interconvertible geometric E-isomer in rat plasma by LC/MS/MS, J. Pharm. Biomed. Anal. 35 (3) (2004) 513–522. [DOI] [PubMed] [Google Scholar]
- [30].Scheffler M, Gion PD, Doroshyenko O, Wolf J, Fuhr U, Clinical pharmacokinetics of tyrosine kinase focus on 4-anilinoquinazolines, Clin. Pharmacokinet 50 (6) (2011) 371–403. [DOI] [PubMed] [Google Scholar]
- [31].Gion PD, Kanefendt F, Lindauer A, Scheffler M, Doroshyenko O, Fuhr U, Wolf J, Jaehde U, Clinical pharmacokinetics of tyrosine kinase inhibitors focus on pyrimidines, pyridines and pyrroles, Clin. Pharmacokinet 50 (9) (2011) 551–603. [DOI] [PubMed] [Google Scholar]
- [32].Thomas-Schoemann A, Blanchet B, Bardin C, Noé G, Boudou-Rouquette P, Vidal M, Goldwasser F, Drug interactions with solid tumour-targeted therapies, Crit. Rev. Oncol. Hematol. 89 (1) (2014) 179–196. [DOI] [PubMed] [Google Scholar]
- [33].van Erp NP, Gelderblom H, Guchelaar HJ, Clinical pharmacokinetics of tyrosine kinase inhibitors, Cancer Treat. Rev. 35 (8) (2009) 692–706. [DOI] [PubMed] [Google Scholar]