Figure 4.
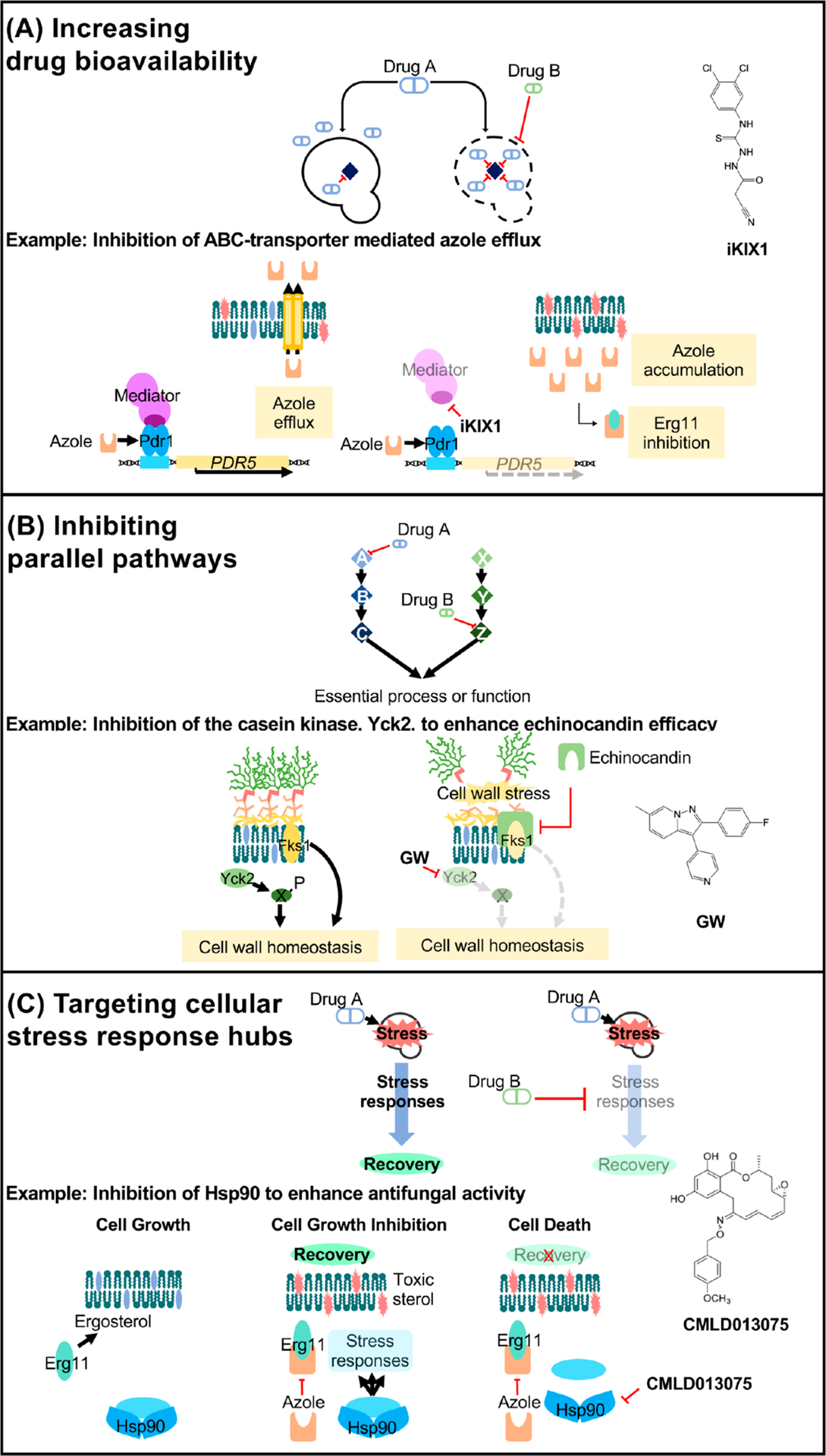
Mechanisms of drug potentiation. (A) Two drugs can potentiate the activity of one another when one drug’s action (drug B) increases the bioavailability of another (drug A) within the target cell. For example, in C. glabrata, the small-molecule iKIX1 (structure shown on right) synergizes with the azoles and resensitizes resistant isolates to fluconazole. iKIX1 disrupts the interaction between the KIX domain within the Gal11/Med15 mediator complex (dark purple) and Pdr1, which prevents upregulation of genes encoding efflux pumps (such as PDR5) in response to the azoles. (B) Two drugs targeting proteins of parallel pathways that converge on a single, essential process or function may also display potentiation activity. Pharmacological inhibition of the nonessential stress kinase Yck2 with the novel 2,3-aryl-pyrazolopyridine compound, GW, potentiates echinocandin efficacy in C. albicans and exacerbates echinocandin-mediated cell wall disruption. (C) Fungal stress responses play a major role in basal antifungal tolerance as well as resistance. Hsp90 is a global regulator of stress-response circuitry, promoting tolerance and resistance to drug-induced stress. Key regulators of cellular stress responses (light blue) are stabilized by Hsp90 in response to antifungal treatment, enabling signal transduction that is necessary to survive in the presence of a drug-induced stress. Pharmacological compromise of Hsp90 using the fungal-selective inhibitor CMLD013075 blocks these signaling networks, thereby preventing the evolution of resistance and abrogating resistance once it has evolved.
