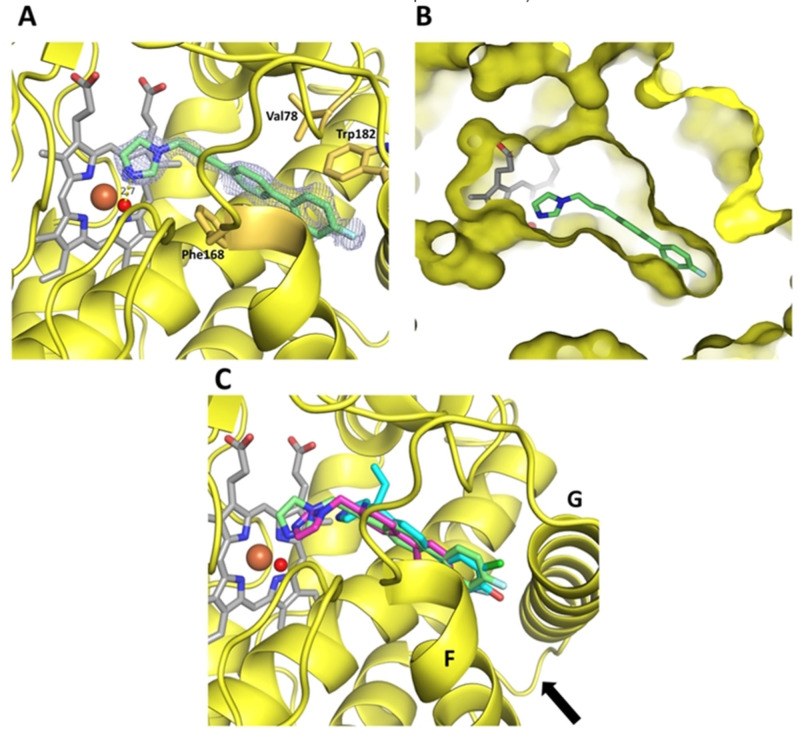
Figure 3.
Complex crystal structures of CYP121. A Coordination of inhibitor L21 in the CYP121 crystal structure. The protein is shown as a yellow cartoon, inhibitor, heme b and interacting residues are shown as sticks. The heme iron is shown as a brown, the water ligand as a red sphere. The difference electron density map of L21 (FO–FC) was contoured at 3σ with phases calculated from a model that was refined in the absence of L21 and is shown as a blue isomesh. The hydrogen bond distance between the water ligand and the imidazole nitrogen is 2.7 Å and shown as a yellow dashed line. B Clipped surface representation of the CYP121‐L21 complex, highlighting the position of the active site heme b and inhibitor L21 in the crystal structure. C Overlay of the complex structures of L21 (green), L44 (magenta) and S2 (cyan). A black arrow is highlighting the location of the entrance to the CYP121 active site.