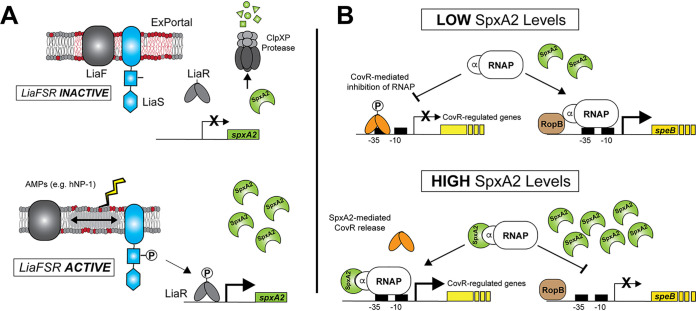
FIG 5.
Model of SpxA2-mediated CovR-regulated gene expression in GAS. (A) Previous studies (19) showed an intimate relationship between the GAS ExPortal, LiaF, and LiaS. Under nonactivating conditions, SpxA2 is rapidly degraded by the ClpXP protease. Disruption of the ExPortal through antimicrobials or antimicrobial peptides (e.g., hNP-1) lead to LiaF/LiaS dissociation and activation of the LiaFSR system (i.e., LiaR phosphorylation [LiaR∼P]). Our data support direct binding of LiaR∼P to the promoter of spxA2, resulting in increased SpxA2 protein—a protein known to alter transcription through direct interaction with the alpha subunit of RNA polymerase (RNAP) (20). (B) When SpxA2 levels are low, GAS cells produce greater amounts of SpeB protease, presumably through increased interaction of RNAP with RopB (regulatory of SpeB expression) (22). In contrast to speB regulation, our data indicate that low-SpxA2 conditions contribute to CovR-regulated gene repression (decreased transcription). However, upon activation of LiaFSR, increased spxA2 transcription leads to increases in the intracellular SpxA2 pool and binding of SpxA2 to RNAP to decrease speB and increase CovR-regulated gene transcription. Thus, SpxA2 may act to alter the regulatory tone of the cell in a regulator- and/or promoter-dependent manner.