FIG 3.
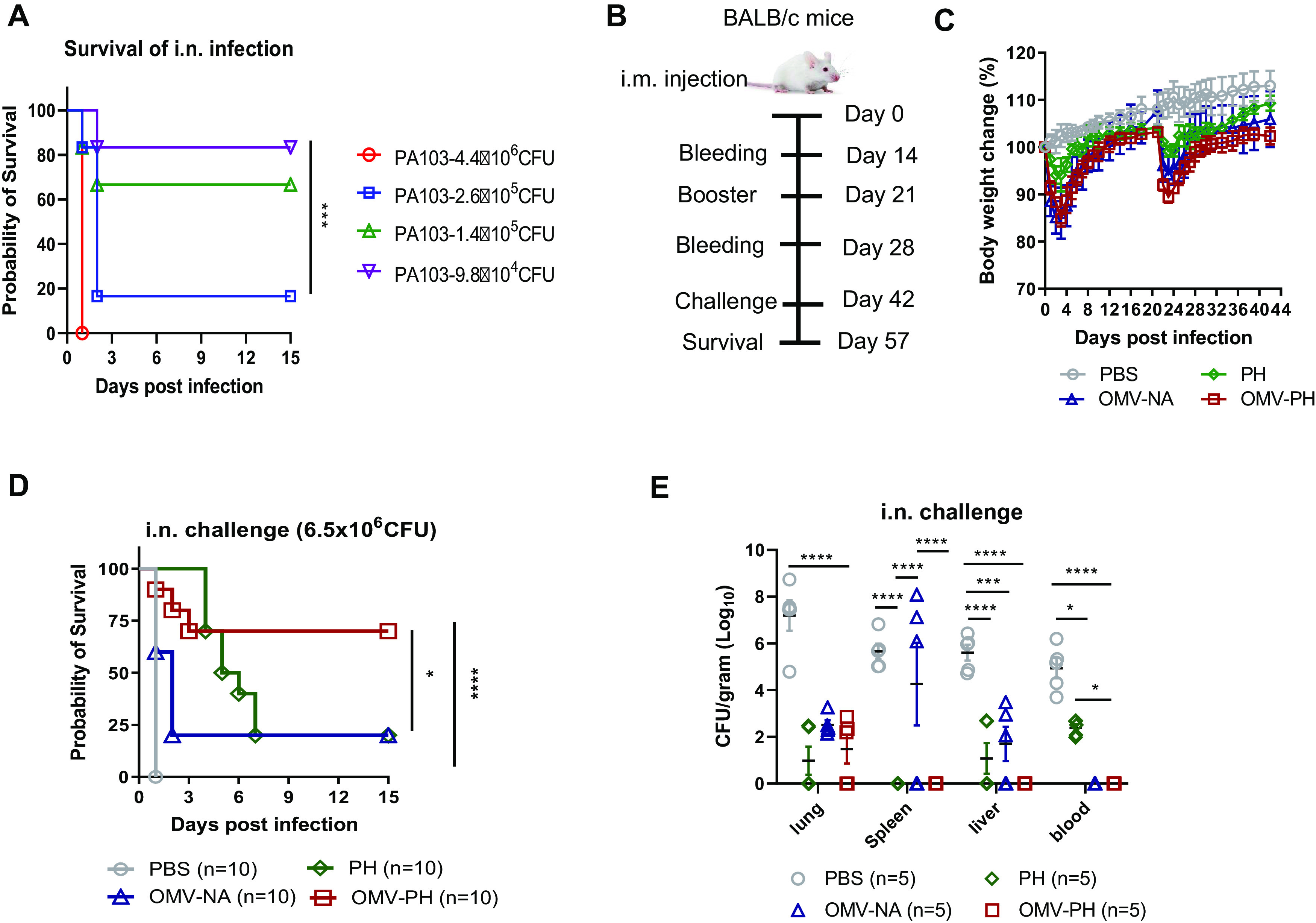
Protective efficacy of P. aeruginosa OMVs against acute pneumonic P. aeruginosa infection. (A) LD50 of intranasal (i.n.) administration. BALB/c mice (n = 10; equal numbers of males and females) were challenged with wild-type PA103 at 4.4 × 106, 2.6 × 105, 1.4 × 105, or 9.8 × 104 CFU/mouse by i.n. administration, and animal survival was recorded for 15 days. (B) Immunization regimen used for the mouse study. (C) BALB/c mice (n = 10) were immunized with either PBS-Alhydrogel, 10 μg of PH-Alhydrogel, 50 μg of OMV-NA, or 50 μg of OMV-PH by i.m. injection and were boosted 21 days after the prime immunization. Mouse weight was monitored and recorded for 6 weeks. (D) On day 42 after the initial immunization, mice were challenged with 6.5 × 106 CFU of wild-type PA103 (∼30 LD50) by i.n. administration, and animal survival was recorded for 15 days. The experiments were performed twice, and data were combined for analysis. Statistical significance was analyzed by the log rank (Mantel-Cox) test. (E) On day 42 after the initial immunization, BALB/c mice (n = 5) were infected i.n. with a sublethal dose (5 × 105 CFU) of PA103. On day 2 postchallenge, different tissues (lung, liver, spleen, and blood) were collected from euthanized mice. Data are shown as means ± SD. The experiments were performed twice, and data were combined for analysis. The statistical significance of differences among the groups was analyzed by two-way multivariant ANOVA with a Tukey post hoc test (ns, no significance; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
