Abstract
Introduction
Data regarding efficacy of second-generation basal insulins (BI) using continuous glucose monitoring (CGM) come from clinical trials. We evaluated the effectiveness of insulin glargine 300 U/ml (Gla-300) compared to insulin degludec 100 U/ml (IDeg-100) in terms of percentage of time in range (TIR); 70–180 mg/dl was obtained from CGM in sub-optimally controlled patients with type 1 diabetes (T1D) in routine clinical practice.
Methods
This observational, multicenter, cross-sectional study included patients with T1D (> 3 years diabetes duration, HbA1c ≥ 7.5%) who had switched from first-generation BI to Gla-300/IDeg-100 within the past 24 months according to physician discretion. Clinical and laboratory data were obtained from clinical records and during study visit, and CGM data were collected prior to the visit.
Results
One hundred ninety-nine people with T1D were included [42.6 ± 13.4 (mean ± SD) years, 18.4 ± 10.4 years diabetes duration]; 104 received Gla-300, 95 IDeg-100. TIR 70–180 throughout whole day was similar in both groups, 52.4 ± 14.0 vs. 49.3 ± 13.9% Gla-300/IDeg-100, respectively. At night, TIR 70–180 and TIR 70–140 were significantly higher in the Gla-300 group compared to the IDeg-100 (52.4 vs. 46.2 and 31.8 vs. 26.9%, respectively, p = 0.0209 and p = 0.0182), and time above range (180) was significantly lower in the Gla-300 group (40.1% vs. 47.2%, p = 0.0199). Additional CGM glucometric data were comparable in both groups. Patient treatment satisfaction score assessed through the Diabetes Treatment Satisfaction Questionnaire (DTSQ) was high and similar for both insulins.
Conclusion
This real-world study shows the effectiveness and safety of Gla-300 are more similar to than different from IDeg-100, with a slightly better nocturnal glucose profile, in sub-optimally controlled T1D patients switching from a first-generation BI.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01153-4.
Keywords: CGM, Gla-300, Glycemic control, T1D
Key Summary Points
| Why carry out this study? |
| Effectiveness of Gla-300 compared to other basal insulins evaluated with continuous glucose monitoring (CGM) is unknown in sub-optimally controlled persons with type 1 diabetes (T1D) |
| Most of the information available on the efficacy of using new insulins and CGM comes from clinical trials conducted under optimal conditions or in highly selected groups of patients |
| OneCARE is a real-world study with time in range as primary endpoint |
| What was learned from the study? |
| The effectiveness and safety of Gla-300 are more similar to than different from IDeg-100, with a slightly better nocturnal glucose profile, in persons with long-standing sub-optimally controlled T1D switching from first-generation BI in routine clinical practice |
| Switch from first- to second-generation basal analogs led to a significant reduction in confirmed hypoglycemia and severe hypoglycemia episodes with no significant differences between Gla-300 and IDeg-100 |
Introduction
Diabetes mellitus (DM) is one of the leading causes of mortality and major morbidities, including cardiovascular disease (CVD), kidney disease (diabetic nephropathy), amputations and blindness. Such complications can be avoided if blood glucose levels remain close to normal glycemic levels [1]. Hypoglycemia is the complication most frequently associated with the treatment of type 1 diabetes (T1D) and one of the main challenges to achieving treatment goals. Achieving glycemic objectives recommended by scientific societies is accomplished in less than a third of patients with T1D [2]. Use of the second-generation basal insulin analogs [3] with a longer duration of action (up to 24 h), flatter action profiles and less day-to-day variability [4] and the incorporation of technology for monitoring glucose profiles can be an advantage for improving glycemic targets without increasing the risk of hypoglycemia.
There are currently two different second-generation basal insulins: insulin glargine 300 U/ml (Gla-300) and insulin degludec (IDeg). Gla-300 uses subcutaneous precipitation as a retarding principle providing consistent activity and extended duration of action. All these properties provide a more constant and prolonged pharmacokinetic and pharmacodynamic profile, > 24 h blood glucose control with less glycemic variability, a lower risk of hypoglycemia and reduction in glycated hemoglobin A1c (HbA1c), as observed in clinical trials [5–12] and real-world studies [13–15], versus Gla-100.
IDeg has an ultralong duration of action and a long half-life with a flatter and stable glucose-lowering effect. IDeg's effect is based on the formation of soluble multi-hexamers in subcutaneous tissues. This creates a reservoir from which monomers are released continuously and slowly to be finally absorbed into the blood flow. It has been demonstrated to have a stable pharmacodynamic profile, which leads to lower fluctuations in glucose levels as also observed in clinical trials [16] and real-world studies [17, 18].
Since the beginning of the century, it has been possibile to measure glucose concentration using portable and minimally invasive continuous glucose monitoring (CGM) systems for 24 h. Within the different types of CGM, those called interactive or “real-time CGM” can allow patients and healthcare professionals to have access to glucose levels, fluctuations in glucose levels and real-time alerts about impending hypo- or hyperglycemia. There are two types of real-time CGM systems: those that provide glucose information continuously and those that provide the same information intermittently whenever the reading monitor approaches the transmitter (intermittent-like, on demand or “flash”-like). Use of these devices has shown benefits in terms of glycemic control, improving the quality of life and helping to reduce HbA1c and mean glucose in patients with DM [19–22].
Limited evidence is available in the T1D population using CGM and about the comparison between first- and second-generation basal insulins and between the two second-generation basal insulins. A current study conducted by Miura et al. comparing the effects of IDeg and Gla-300 on glycemic stability in T1D patients using CGM concluded that both insulins were comparable in terms of glucose-stabilizing effects [23]. These results were also observed in terms of glycemic control in the type 2 diabetes (T2D) population when comparing Glar-300 and IDeg using a CGM device [24]. However, most of the evidence on the efficacy of using second-generation basal insulins and CGM comes from clinical trials and real-world studies conducted under optimal conditions or in highly selected groups of patients.
The main objective of the ONECARE study was to describe the effectiveness and safety of Gla-300 versus Degludec 100U (IDeg-100), defined as the percentage of time in range (TIR) (glucose 70–180 mg/dl) during 14 consecutive days within a 4-week period measured using CGM in patients with suboptimally controlled T1D in routine clinical practice in Spain.
Methods
Study Design
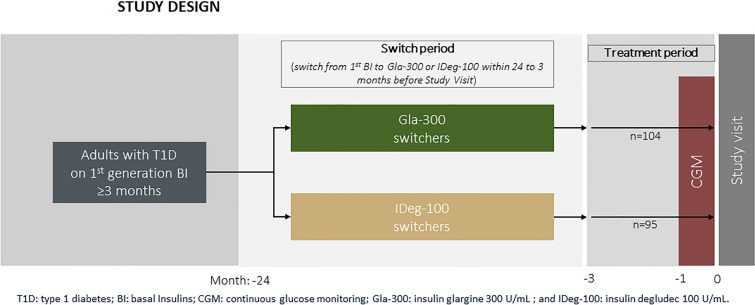
This was an observational, retrospective, cross-sectional, multicenter study conducted in endocrinology departments of 21 hospitals in Spain (Supplementary Material Table S1), including adults with T1D who had switched from a first-generation basal insulin (BI) analog (insulin glargine 100 U/ml or detemir) to a second-generation basal insulin, either Gla-300 or IDeg-100, within 24 months of the study visit. The study comprised two phases (Fig. 1): (1) the period in which patients were treated with basal bolus (BB) insulin (first generation BI) for a minimum of 3 months [patients on intermediate acting (NPH) and premixed insulin were excluded]; (2) the period when the patients, as per physician criteria, switched the BI, within the last 24 months before the study visit, to either Gla-300 or IDeg-100. CGM was performed using the Freestyle Libre® CGM system (Abbott Diabetes Care, Witney, UK), and data from 14 consecutive days within the last month were analyzed, the period recommended by international consensus to assess the glycemic profile and enable decision-making in clinical practice [25].
Fig. 1.
Study design
The inclusion criteria were adults diagnosed with T1D at least 3 years prior to study enrollment; switched from ≥ 3 months of treatment with BB insulin treatment (first-generation BI) to Gla-300 or IDeg-100 within the previous 24 months; having HbA1c ≥ 7.5% before the switch; maintaining current treatment ≥ 3 months; using a CGM device for at least 1 month prior to enrollment in the study; having at least 70% of useable CGM data available.
Exclusion criteria included: using an insulin pump; using NPH or premixed insulin (mixture of NPH and rapid insulin) prior to or after the switch; treatment with non-insulin antidiabetic agents; having received or being treated with oral or injectable corticosteroids; receiving > 80 U/day of BI analogs and/or not receiving a stable dose (± 20% of the total dose) within 30 days prior to inclusion; having fewer than two injections of fast-acting insulin analogs per day within 30 days prior to inclusion.
CGM data were obtained from the Freestyle Libre®, while sociodemographic, clinical (treatment, laboratory) and safety data were collected retrospectively from the medical records and directly from the patients during the study visit in which two patient-reported outcomes (PRO) were also collected using the Diabetes Treatment Satisfaction Questionnaire (DTSQ) [26] and Insulin Treatment Satisfaction Questionnaire (ITSQ) [27]. Hypoglycemia awareness was evaluated using the Spanish-validated version of Clarke’s questionnaire (a score < 3 was considered normal perception) [28].
The study protocol was approved by the Spanish Agency of Medicines and Medical Devices and by the Ethics Committee of the Hospital Clinic of Barcelona, Spain (reference no. HCB/2018/0563). All procedures were performed in accordance with the Helsinki Declaration of 1964 and its later amendments and conformed with national regulations applicable during the study (Order SAS/3470/2009 of 16 December 2009, which published the guidelines on observational studies on medicinal products for human use post-authorization). All patients provided written informed consent for participation in this study.
Endpoints
The primary endpoint was the percentage of time within the predefined CGM glucose range TIR of 70–180 mg/dl [complete day, night (24:000–05:59) or day (06:00–23-59) period] during 14 consecutive days within a 4-week period with CGM data obtained from the FreeStyle Libre®.
Secondary glucometric endpoints based on CGM data included other ranges in the full day, daytime and nighttime periods: TIR 70–180 mg/dl; TIR 70–140 mg/dl; time above range (TAR) > 180 mg/dl and > 250 mg/dl; time below range (TBR): < 70 mg/dl and < 54 mg/dl, mean glucose profile over 24-h period, percentage of estimated HbA1c, coefficient of variation (CV), interquartile range (IR), area under the curve (AUC), average daily risk range (ADRR), high blood glucose index (HBGI) and low blood glucose index (LBGI) [29], mean amplitude of glycemic excursions (MAGE) and mean daily difference (MODD) in glucose. A hypoglycemic episode was defined as a period of > 15 min duration below a specific threshold. Likewise, a hyperglycemic episode was defined as a period of > 15 min duration above a specific threshold.
Other secondary endpoints collected through medical data were the percentage of patients with hypoglycemic events in the 12 months before the switch versus events after the switch to study visit, mean HbA1c and fasting plasma glucose (FPG), and number of adverse events. A confirmed hypoglycemia event was defined as an episode of symptomatic or asymptomatic hypoglycemia with plasma glucose < 54 mg/dl or < 70 mg/dl, and a severe hypoglycemia event was defined as an episode of hypoglycemia at any time of the day (24 h) and during the night that requires assistance from another person administering carbohydrates, glucagon, or any other corrective measure.
Statistical Analyses
The sample size of 214 patients (107 patients per group) allowed comparison of TIR 70–180 mg/dl between patients with Gla-300 and those with IDeg-100. A minimum difference of 3.3% was considered (difference between mean percentage of time within a glucose range of 70–180 mg in Gla-300 and other basal insulin of 57.8% and 54.5%, respectively, in the Bergenstal et al. study [5]) with a significance level of 0.05 and a statistical power of 0.80.
The analysis of the primary and some secondary objectives (those related to data obtained from the FreeStyle Libre® device) was performed by the HealthPartners Institute, International Diabetes Center (IDC), in Minnesota. The comparison of results between the two study groups, facilitated by the IDC, was performed using IQVIA Information S.A.
Percentage of time in TIR was compared between groups with Student’s t-test. The secondary endpoints were tested for significance as intergroup differences of normally or non-normally distributed data with the unpaired Student's t-test or Mann-Whitney U-test, respectively. A two-sided p value of < 0.05 was considered statistically significant for all other analyses. Percent of patients with hypo- and hyperglycemic episodes was compared between groups with the chi-square test, and McNemar's Test was used for the comparison between serious events before and after the treatment.
Continuous variables were described by the number of patients with valid observations, mean and standard deviation (SD). Categorical variables were described by number and percentages of patients per response category.
Statistical analyses were generated using SAS software, version 7.15, Enterprise Guide or above.
Results
Patients and treatment characteristics
A total of 220 participants were included in the study, and 21 were excluded for not having 70% CGM data from the Freestyle Libre® device for 14-consecutive days within the last month prior to inclusion in the study. Of the 199 valid patients, 104 (52.3%) switched to Gla-300 and 95 (47.7%) to IDeg-100.
Sociodemographic and clinical characteristics (BMI, height, blood pressure and heart rate) were similar between the two groups, as shown in Table 1 and in the Supplementary Material Table S2. Hypercholesterolemia (23.6%), hypertension (12.1%) and diabetic retinopathy (20.6%) were the most frequent comorbidities observed in both groups, the proportion of patients with retinopathy being statistically higher in the IDeg-100 group than in the Gla-300 group (27.4% vs. 14.4%, p = 0.0241). Mean time since T1D diagnosis was 18.4 ± 10.4 years overall, but it was significantly shorter in the Gla-300 group than in the IDeg-100 group: 16.8 ± 10.2 vs. 20.2 ± 10.5 years (p = 0.0218), respectively.
Table 1.
Participant characteristics
| Total N = 199 |
GLA-300 N = 104 |
IDEG-100 N = 95 |
p value | |
|---|---|---|---|---|
| Age years | 42.6 ± 13.4 | 43.5 ± 14.3 | 41.7 ± 12.3 | 0.3500 |
| Women, n (%) | 100 ± 50.3 | 46 ± 44.2 | 54 ± 56.8 | 0.0755 |
| BMI, kg/m2 | 26.0 ± 3.8 | 26.2 ± 4.0 | 25.7 ± 3.6 | 0.4425 |
| Time since T1D diagnosis, years | 18.4 ± 10.4 | 16.8 ± 10.1 | 20.2 ± 10.5 | 0.0218 |
| Comorbidities Associated to T1D, n (%) | 99.0 ± 49.7 | 45 ± 43.3 | 54 ± 56.8 | 0.0558 |
| Dyslipidemia | 47 ± 23.6 | 23 ± 22.1 | 24 ± 25.3 | 0.6015 |
| Hypertension | 24 ± 12.1 | 14 ± 13.5 | 10 ± 10.5 | 0.5254 |
| Retinopathy | 41 ± 20.6 | 15 ± 14.4 | 26 ± 27.4 | 0.0241 |
| Nephropathy | 12 ± 6.0 | 5 ± 4.8 | 7 ± 7.4 | 0.4485 |
| Hypothyroidism | 19 ± 9.5 | 9 ± 8.7 | 10 ± 10.5 | 0.6535 |
| Total daily insulin dose, U/kg/day | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.1456 |
| Fast-acting insulin, n (%) | ||||
| Lispro (Humalog®) | 51 2 ± 5.6 | 23 ± 22.1 | 28 ± 29.5 | 0.5310 |
| Aspart (Novorapid®) | 106 ± 53.3 | 56 ± 53.8 | 50 ± 52.6 | |
| Glulisina (Apidra®) | 36 ± 18.1 | 22 ± 21.2 | 14 ± 14.7 | |
| Regular (Actrapid®, Humulina regular®) | 6 ± 3.0 | 3 ± 2.9 | 3 ± 3.2 | |
| Basal insulin, n (%) | ||||
| Detemir (Levemir®) | 18 ± 9.0 | 7 ± 6.7 | 11 ± 11.6 | 0.2758 |
| Glargine 100 U/ml (Lantus®) | 180 ± 90.5 | 97 ± 93.3 | 83 ± 87.4 | |
| Glargine 100 U/ml (Abasaglar®) | 1 ± 0.5 | 0 | 1 ± 1.1 |
Data are mean ± SD unless otherwise stated
BMI: body mass index; T1D, type 1 diabetes
No significant differences were observed in the number of months that the patients were on the BI, either Gla-300 or IDeg-100, at the time of the study, with time elapsed between the change in insulin and the study visit being 14.5 ± 6.6 months (14.3 ± 6.6 vs. 14.7 ± 6.6 months for Gla-300 and IDeg-100, respectively; p = 0.6908). BI was most often administered in the evening in both study groups (in 58% of participants before the switch vs. 60% after the switch). There were no significant changes in the daily time schedule of BI administration after changing BI in both groups. The most frequent reasons why physicians decided to change the patient's BI were a suboptimal HbA1c in 52.3% of patients and recurrent hypoglycemia in 49.2%, and no differences were observed between the two groups.
Before the switch, 90.5% of T1D patients were treated with Gla-100, 25.4 ± 12.5 UI/day, 9.0% with Detemir and 0.5% (1 patient) with Gla-100 biosimilar (Abasaglar®). The total daily insulin dose per kilogram body weight was similar between study groups at the time of the study visit (0.7 U/kg/day for Gla-300 vs. 0.6 U/kg/day for IDeg-100; p = 0.1465). At the study visit, mean Gla-300 dose was significantly higher, 28.5 ± 12.6 UI/day compared to IDeg-100, 22.8 ± 10.7 (p = 0.0010). No difference in prandial insulin dose was observed between Gla-300 and IDeg-100 (22.2 ± 13.8 vs. 21.8 ± 11.6; p = 0.8199) respectively.
The proportion of patients with normal hypoglycemia awareness measured using Clarke’s questionnaire was not different between the study groups (65% of the participants).
Endpoints Obtained from CGM
Primary Endpoint
Effectiveness Measured as TIR 70–180 mg/dl
The CGM device was active a mean time percentage of 93.5% for both arms. The mean percentage of time within TIR 70–180 mg/dl within the last 14-consecutive days for the full day period was similar between the Gla-300 and IDeg-100 groups: 52.4 ± 14.0% versus 49.3 ± 13.9% (Table 2 and Supplementary Material Figure S1). The same was observed in TIR 70–140 mg/dl.
Table 2.
Glucometric data of patients using either GLA-300 OR IDEG-100
| Total n = 199 |
Gla-300 n = 104 |
IDeg-100 n = 95 |
p value | |
|---|---|---|---|---|
| Mean glucose over 24-h period (mg/dl) | 175.1 ± 31.6 | 171.6 ± 31.6 | 178.9 ± 31.3 | 0.1032 |
| Estimated HbA1c (%) | 7.7 ± 1.1 | 7.6 ± 1.1 | 7.9 ± 1.1 | 0.1032 |
| TIR (%) | ||||
| 70–180 mg/dl | 50.9 ± 14.0 | 52.4 ± 14.0 | 49.3 ± 13.9 | 0.1191 |
| 70–140 mg/dl | 30.9 ± 10.7 | 31.9 ± 11.0 | 29.7 ± 10.2 | > 0.05 |
| TAR (%) | ||||
| > 180 mg/dl | 42.8 ± 15.9 | 41.0 ± 16.1 | 44.8 ± 15.6 | 0.0935 |
| > 250 mg/dl | 16.8 ± 13.4 | 15.4 ± 13.0 | 18.2 ± 13.8 | 0.1453 |
| TBR (%) | ||||
| < 54 mg/dl | 2.3 ± 3.2 | 2.5 ± 3.7 | 2.2 ± 2.7 | 0.4433 |
| < 70 mg/dl | 6.3 ± 5.4 | 6.6 ± 6.0 | 5.9 ± 4.7 | 0.3663 |
| SD (mg/dl) | 69.5 ± 15.0 | 67.8 ± 15.4 | 71.3 ± 14.4 | 0.0934 |
| CV of glucose levels (%) | 39.9 ± 6.8 | 39.8 ± 7.1 | 40.1 ± 6.5 | |
| < 36% (stable) | 29.1% | 32.7% | 25.3% | 0.6903 |
| ≥ 36% (instable) | 70.9% | 67.3% | 74.7% | 0.2494 |
| AUC (mg/dl) complete day | 4098.3 ± 780.7 | 4008.4 ± 773.8 | 4196.7 ± 780.5 | 0.0893 |
| LBGI | 1.5 ± 1.4 | 1.6 ± 1.6 | 1.4 ± 1.2 | 0.3076 |
| HBGI | 4.6 ± 2.4 | 4.4 ± 2.4 | 4.9 ± 2.5 | 0.1215 |
| ADRR | 48.4 ± 11.8 | 47.4 ± 12.2 | 49.5 ± 11.3 | 0.1980 |
| MAGE 24 h (mg/dl) | 153.1 ± 33.2 | 149.4 ± 34.4 | 157.2 ± 31.6 | 0.1006 |
| MODD in glucose (mg/dl) | 68.4 ± 16.7 | 66.4 ± 18.0 | 70.5 ± 14.9 | 0.0822 |
Data are mean ± SD unless otherwise stated. Glucometric data analyzed in the full day period
TIR: time in range, TAR: time above range, TBR: time below range, SD: standard deviation, CV: coefficient of variation, AUC: area under the curve, LBGI: low blood glucose Index, HBGI: high blood glucose index, ADRR: average daily risk range, MAGE: mean amplitude of glycemic excursions, MODD: mean of daily difference
Secondary Endpoints
Time at Glucose Target Levels Measured Through CGM
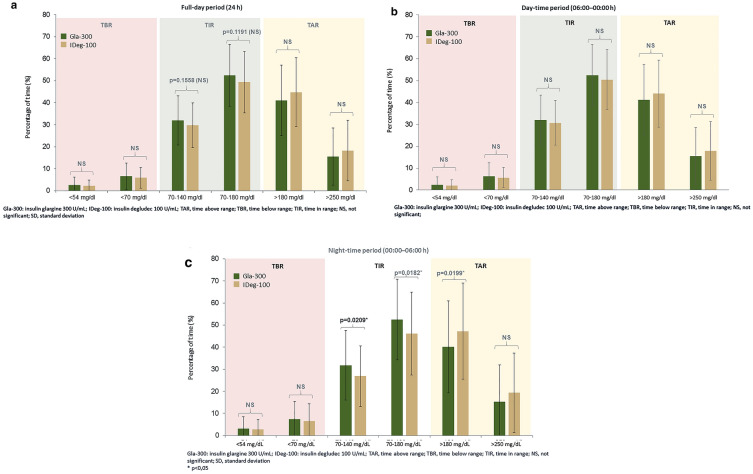
There was no significant difference in TIR, TBR and TAR for the full day period between the two treatment groups, as shown in Fig. 2a, b and Table 2.
Fig. 2.
Percentage of time at glucose target levels for different periods during 24 h
When analyzing the day and night periods, significant differences between the two groups were observed in TIR 70–140 mg/dl, TIR 70–180 mg/dl and TAR > 180 mg/dl in the nocturnal period (24:00–06:00), as shown in Fig. 2c, with no significant differences observed in the day period. Those patients treated with Gla-300 presented a higher percentage in the recommended range of 70–180 mg/dl (52.4% Gla-300 vs. 46.2% IDeg-100, p = 0.0182) and 70–140 mg/dl (31.8% Gla-300 vs. 26.9% IDeg-100, p = 0.0209) and a lower percentage in TAR > 180 mg/dl (40.1% Gla-300 vs. 47.2% IDeg-100, p = 0.0199).
Additional Glucometric Values from CGM
No significant differences between groups in the full day period were observed in additional data obtained from CGM including glycemic variability metrics, as shown in Table 2. Mean 24-h glucose curves for the Gla-300 group were significantly lower (lower glycemic excursions) at night than for IDeg-100 patients (170.4 mg/dl vs. 181.9 mg/dl; p < 0.05) (data not shown).
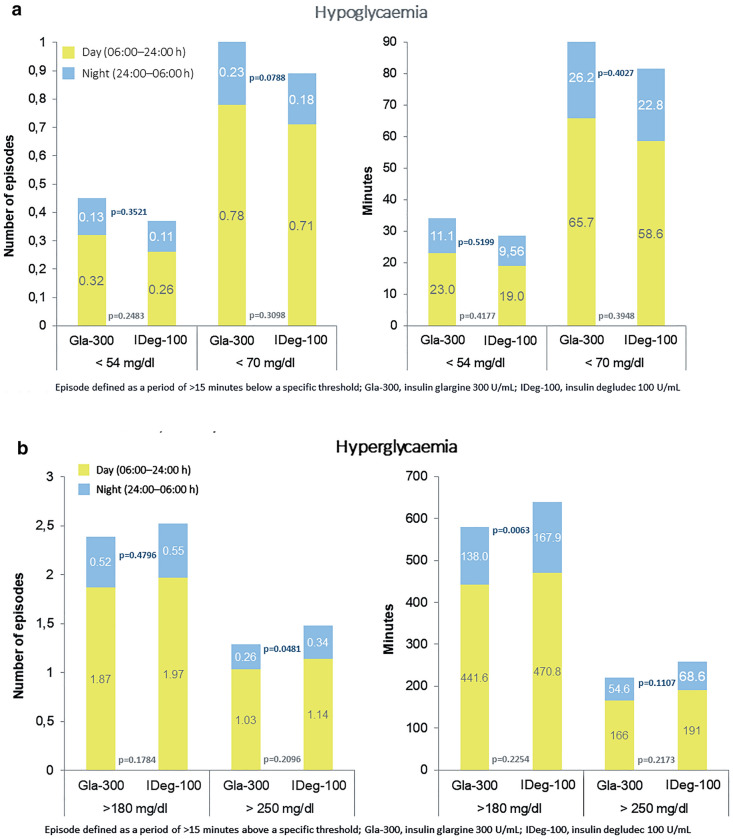
Number of Episodes and Minutes in Hypoglycemia
No significant differences in the average number of hypoglycemic episodes and in the average minutes per day spent in hypoglycemia (< 70 and < 54 mg/dl) in the 14 consecutive days of CGM prior to study visit were observed between study groups in any of the periods analyzed (complete day, night or day period) (Fig. 3a).
Fig. 3.
Average number of episodes per day and average minutes per day of episodes in a hypoglycemia and b hyperglycemia
Number of Episodes and Minutes in Hyperglycemia
No significant differences in the number of hyperglycemic episodes and in the average minutes per day spent in hyperglycemia (> 180 mg/dl) in the 14-consecutive days prior to study visit were observed between study groups in either the complete day or daytime period. This remained the same when the number and minutes of hyperglycemia episodes (> 250 mg) were analyzed for these same periods of time. Nevertheless, during the nighttime period, the number of episodes (> 250 mg/dl) per day was significantly lower in the Gla-300 group (Fig. 3b). Likewise, in terms of average minutes per day spent > 180 mg/dl, it was observed that Gla-300-treated patients spent fewer minutes per day in hyperglycemia during the nighttime period (Fig. 3b).
Endpoints Obtained from Clinical Records and at the Study Visit
HbA1c and FPG values obtained in the 4 months prior to the study visit were available in 88.0% of the study population: 74.9% in the last 2 months and 13.1% in the 2–4 months prior to the visit. The mean HbA1c value closest to the study visit was similar in both groups: 7.8% ± 1.0 in Gla-300 and 8.0% ± 0.9 in IDeg-100 (p = 0.2529). The proportion of patients achieving a HbA1c < 7% was higher in Gla-300 compared with the IDeg-100 group (Table 3).
Table 3.
HBA1C, FPG and hypoglycemic events according to the medical record
| Before the switch | At the study visit | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Gla-300 | IDeg-100 | p value | Total | Gla-300 | IDeg-100 | p value | |
| HbA1c % (1) | 8.4 ± 1.1 | 8.3 ± 0.9 | 8.6 ± 1.2 | 0.0680 | 7.8 ± 1.0 | 7.8 ± 1.0 | 8.0 ± 0.9 | 0.2529 |
| HbA1c < 6.5%, % | 0 | 0 | 0 | – | 2.7 | 4.2 | 1.1 | 0.2112 |
| HbA1c < 7%, % | 0 | 0 | 0 | – | 13.7 | 18.8 | 8.0 | 0.0352 |
| Mean FPG, mg/dl (1) | 172.9 ± 70.2 | 177 ± 70.7 | 168.5 ± 69.9 | 0.4530 | 151.3 ± 67 | 155.9 ± 65.4 | 146.1 ± 68.9 | 0.3519 |
| Hypoglycemic events* < 70 mg/dl, n (2) | 10.5 ± 13.6 | 11.8 ± 16.1 | 8.4 ± 7.8 | 0.8042 | 7.9 ± 10.1 | 8.3 ± 11.8 | 7.3 ± 6.9 | 0.3488 |
| Hypoglycemic events* < 54 mg/dl, n (2) | 4.9 ± 8.1 | 4.7 ± 8.1 | 5.2 ± 8.4 | 0.5761 | 3 ± 5.6 | 2.9 ± 5.3 | 3.2 ± 6.2 | 0.7544 |
| Severe hypoglycemic events**, n (2) | 0.3 ± 1.4 | 0.5 ± 1.9 | 0.2 ± 0.7 | 0.7002 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.6327 |
Data are mean ± SD unless otherwise stated
HbA1c hemoglobin A1c, FPG fasting plasma glucose
*Confirmed hypoglycemia defined as an episode of symptomatic or asymptomatic hypoglycemia with plasma glucose < 54 mg/dl (3.0 mmol/l) or < 70 mg/dl (3.9 mmol/l)
**Severe hypoglycemia is defined as an episode of hypoglycemia at any time of the day (24 h) and during the night that requires assistance from another person administering carbohydrates, glucagon, or any other corrective measure
(1) HbA1c and FPG were collected in the 4 months prior to the switch to Gla-300 or IDeg-100 and in the 4 months prior to study visit
(2) Hypoglycemic events were collected in the 12 months before the switch and in the period from switch to study visit [time elapsed between switch and the study visit of 14.5 months (6.6)]
In the total population, it was observed that patients with HbA1c < 7 had a better nocturnal TIR (TIR 70–180 mg/dl and TIR 70–140 mg/dl) than patients with HbA1c > = 7% (59.4% of the time in TIR 70–180 mg/dl vs. 47.5% of the time in TIR 70–180 mg/dl, p = 0.0028 and 38.21% of the time in TIR 70–140 mg/dl vs. 27.7% of the time in TIR 70–140 mg/dl, p = 0.0010).
A relation was observed in the Gla-300 group between a better nocturnal TIR (70–140 mg/dl and 70–180 mg/dl) and lower HbA1c < 7% level, indicating that Gla-300 has a better nocturnal profile in CGM and confers better glycemic results in real-life clinical practice.
Patients with HbA1c < 7% in the Gla-300 group had a better nocturnal TIR (70–140 mg/dl and 70–180 mg/dl) than patients with HbA1c > = 7% (60.3% of the time in TIR 70–180 mg/dl vs. 50.2% of time in TIR 70–180 mg/dl, p = 0.0324, and 40.3% of the time in TIR 70–140 mg/dl vs. 29.8% of the time in TIR 70–140 mg/dl, p = 0.0103). This relation was not observed in the IDeg-100 group.
Confirmed hypoglycemic events (< 70 mg/dl, < 54 mg/dl and severe hypoglycemia) were obtained during the 12 months prior to switch and in the period from switch to the study visit [median time elapsed between switch and the study visit of 14.5 (percentile 8.3–20.6 months)]. Data regarding confirmed hypoglycemic events < 70 mg/dl and < 54 mg/dl in medical records were available in 40.0% of patients; this figure increased to 82.0% in the case of severe hypoglycemia. A decrease in the number of confirmed hypoglycemia and severe hypoglycemia events after the switch from first-generation BI to second-generation BI was observed, without significant differences between the study groups. Fewer patients had an episode of severe hypoglycemia when treated with a second-generation BI (3.1% vs. 12.9%, second vs. first generation BI, respectively; p = 0.0003).
Likewise, more patient-reported hypoglycemic events were observed (< 70 mg/dl, < 54 mg/dl and severe hypoglycemia) in the 12 months before the change of BI compared to the period from the switch to the study visit. No differences between groups were observed (Table 3).
Results on Diabetes Treatment Satisfaction Questionnaire and Insulin Treatment Satisfaction Questionnaire
The mean global score for DTSQs was 27.8, reflecting high treatment satisfaction with both insulins, without any difference between them (Supplementary Material Figure S2). The same result was obtained from the ITSQ questionnaire, with a mean global score of 67.2 ± 21.5.
Discussion
The OneCARE study provides, for the first time to our knowledge, information regarding the comparison of effectiveness of second-generation basal insulin analogs, Gla-300 and IDeg-100, in terms of CGM-related metrics in patients with T1D previously sub-optimally controlled with first-generation basal insulin analogs. Our real-world study suggests that both BIs are similar in achieving CGM targets according to international consensus, mainly 24-h TIR 70–180 mg/dl. The recent RESTORE real-world retrospective study has also shown that switching from a first-generation BI to Gla-300 or IDeg-100 provided similar improvements in glycemic control and a significant decrease in hypoglycemia [30].
To date, very scarce evidence comparing Gla-300 and IDeg-100 in the T1D population is available. Direct comparison of both insulins is limited to two studies that did not use CGM information [31, 32]. The results regarding pharmacodynamics vary. Recently, Miura et al. reported the effects of both insulins on glycemic stability in c-peptide-negative T1D patients using CGM [23]. In a multicenter randomized crossover design including 46 patients, the authors suggested that Glarg-300 and IDeg-100 have comparable stabilizing effects on the glucose profile. They did not find any significant difference in the percentage of CGM readings in the target glucose ranges with the exception of a decrease in TBR < 70 mg/dl in those patients switched from IDeg-100 to Gla-300.
In this context, and as has been mentioned in a recent review [33], there is a need for studies utilizing CGM in a head-to-head comparison of second-generation BI in T1D. As both types of studies are deemed complementary, this need applies not only to randomized clinical trials but also to real-world pragmatic studies. In this context, a few months ago, Battelino et al. published the study design of the InRange open-label, randomized, parallel trial comparing Gla-300 and IDeg-100 in sub-optimally controlled patients with T1D using CGM and TIR consensus group recommendations for the primary outcome [33]. The hypothesis being tested is that Gla-300 is non-inferior to IDeg-100 concerning the percentage TIR after 12-week follow-up. While we wait for the results of the InRange trial, we think that the information obtained in OneCare is worth of attention.
In the real-world clinical practice scenario, Gla-300 is comparable to IDeg in terms of the overall TIR and safety profile, similar to those found in published studies [34, 35]. However, Gla-300 showed more nocturnal time periods in the TIR range at night in suboptimally controlled T1D patients switching from first-generation BI compared with IDeg.
Our hypothesis is aligned with results observed in the study by Carral et al. [36]. In this retrospective observational study, a higher percentage of patients in the Gla-300 group reached the target of HbA1c ≤ 7,% and more patients treated with IDeg-100 presented HbA1c > 8%. In addition, in a subgroup of 71 patients (17%) who used CGM (FreeStyle Libre), a significant difference was observed in TIR > 180 mg/dl not only in the nighttime period but also throughout the study period.
The reason why Gla-300 has better results could be explained by the different pharmacodynamic profiles of the two drugs. As stated by Bailey et al., Gla-300 provides fewer fluctuating steady-state pharmacodynamic profiles (lower within-day variability) and more evenly distributed pharmacokinetic profiles compared with IDeg-100 [31].
These similar effects on the glucose profile are achieved using more units of Gla-300 than IDeg-100. As previously shown, this is not the result of the lower potency of Gl-300 but of its lower bioavailability after subcutaneous injection [10, 37]. Related to its nature, there were some differences in the clinical characteristics of the two groups of patients receiving the two second-generation insulin analogs. The duration of T1D and presence of retinopathy were longer and higher, respectively, in patients using insulin IDeg-100. However, we think that in a population with, on average, nearly 2 decades since the diagnosis of T1D, the impact of a sparse difference of 3 years could be considered almost negligible. The rest of the clinical, laboratory and treatment characteristics, including pre-switch HbA1c, and the reasons for the switch from a first-generation BI were comparable in the two groups.
Regarding the results achieved by both insulins in terms of CGM-based targets, it should be underlined that in neither TIR nor TBR were the recommendations of the ATTD consensus for CGM utilization achieved [38]. However, this is not surprising considering the clinical characteristics of the participants in the real-world OneCare study: T1D patients with longstanding disease, with poor metabolic control in which may be less stringent A1C goals might be applied [39]. In addition, at the time of inclusion of participants in our study, there was no reimbursement for CGM by the national health care provider. Thus, the use of self-financing CGM by participants could be related to their additional difficulties in obtaining optimal metabolic control despite using BB treatment including second-generation BI. Moreover, this use could also create a bias in the process of patient selection, including patients with more complications and more difficulties managing their glycemic control having more interest in participating in the study and not all of the T1D population being included [40].
Regarding the result obtained from clinical records, in fact, results of the real-world OneCARE study show that both second-generation BIs, Gla-300 and IDeg, performed better than first-generation BI in terms of glucose control and hypoglycemic events, supporting data obtained in the clinical program from both second-generation BIs. In the DELIVER-2 [13] and DELIVER-3 [14] study, Gla-300 showed greater improvements in glycemic control and reduced the risk of hypoglycemia in T2D when switching from Gla-100. Similar effects were found with the IDeg switching as observed in the prospective real-world study of Fadine et al., in which the switch to degludec from another BI was associated with significantly lower rates of hypoglycemia and improved glycemic control [41].
Our study has limitations; we are well aware of them, and some have already been mentioned. The lack of randomization, lack of a centralized laboratory and lack of intensive control and monitoring of all aspects of usual clinical practice are some of the weaknesses of real-world studies that undermines the internal validity of the results. Also, although the diabetes duration difference was only 3 years between groups, the IDeg-100 group presented a more severe course of the disease, (higher rates of retinopathy). Nevertheless, a post-hoc analysis has been performed aiming to assess the effect of T1D duration and the presence or absence of retinopathy in both arms of the study, and it was observed that the equivalent effectiveness of Gla-300 and IDeg-100 in sub-optimally controlled T1D patients switching from a first-generation basal insulin seems not to be affected by either the duration of the disease or the presence or absence of retinopathy (data not shown). The results of this analysis are in line with the results observed in the main results of the study. However, the strength of the study is, to the best of our knowledge, that this is one of the first studies comparing head-to-head Gla-300 and IDeg-100 effectiveness in clinical practice in a large sample size of sub-optimally controlled T1D patients using CGM metrics demonstrating the similarities of both insulins after the assessment of outcomes based on objectively collected data.
Conclusion
In summary, in terms of the achievement of CGM-based targets from the last consensus recommendations, this real-world study suggests that the effectiveness and safety of Gla-300 insulin is similar to those obtained with IDeg-100 in patients with long-standing sub-optimally controlled T1D switching from first-generation BI.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This project was supported by Sanofi, which was involved in the study design; in the collection, analysis and interpretation of the data; and in the decision to submit the paper for publication. Medical writing support was provided by Anna de Prado from IQVIA Information S.A. Spain, and the journal’s Rapid Service Fee was funded by Sanofi.
Medical Writing and Editorial Assistance
We thank all participating physicians for their dedication to participant care and Anna de Prado and Neus Canal from IQVIA Information S.A Spain for conducting the study, medical writing the statistical analysis.
Prior Presentation
Data included in this manuscript were presented, in part, at the 56th European Association for the Study of diabetes (EASD), Virtual Annual Meeting 2020 and in Advanced Technologies & Treatments for Diabetes (ATTD), Virtual Meeting 2021.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Authorship Contributions
Ignacio Conget contributed to the study design, conduct/data collection, analysis and writing of the manuscript. Mireia Borrell contributed to the study design, analysis and writing the manuscript. The remaining authors contributed to the conduct of the study/data collection and writing of the manuscript. All authors gave final approval of the version to be published, participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspect of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures
Ignacio Conget—Received a fee from Sanofi Aventis for coordination of the OneCARE study, and received lecturing and consulting fees from Medtronic, Bayer, GlaxoSmithKline, Eli Lilly, Novo Nordisk, Sanofi Aventis, Novartis, AstraZeneca and MSD. Miguel Ángel Mangas—Received consulting fees and/or honoraria for training activities, courses or advisory meetings Sanofi, Novo Nordisk, Eli Lilly, AstraZeneca, Boehringer Ingelheim, Esteve Janssen, Abbot, and has received honoraria for participation as a researcher in clinical trials Sanofi, Novo Nordisk, AstraZeneca, GlaxoSmithKline, Millendo Therapeutics. Cristóbal Morales—Clinical Trials: Novo Nordisk, Sanofi, AstraZeneca, Pfizer, Lilly, Merck, Lexicon, FPS, Hanmi Janssen, Boehringer Ingelheim, Takeda, Roche, Theracos LeeGanz Advisory board Novo Nordisk, Eli Lilly, MSD, Boehringer Ingelheim, AstraZeneca, Sanofi, Abbott Speaker Sanofi, Novo Nordisk, AstraZeneca, Roche, Eli Lilly, Boehringer Ingelheim, MSD, Ferrer Pharma, Janssen, Abbot. Juan Caro -Reports no disclosures. Margarita Gimenez—Received lecturing and consulting fees from Medtronic, Eli Lilly, Novo Nordisk, Sanofi Aventis, AstraZeneca and MSD. Mireia Borrell—Employed by and has ownership interest in Sanofi. Elías Delgado—Received unrestricted research support from AstraZeneca, Novo Nordisk, Sanofi, Pfizer, and Roche, and has received consulting fees and/or honoraria for membership on advisory boards and speaker’s bureau from AstraZeneca, Novo Nordisk, Eli Lilly, Sanofi, GlaxoSmithKline, Pfizer, Almirall Novartis, Abbott Laboratories, Esteve and MSD.
Compliance with Ethics Guidelines
The authors state that they have received approval from the Spanish Agency of Medicines and Medical Devices and by the Ethics Committee of the Hospital Clinic of Barcelona (reference no. HCB/2018/0563). All procedures were performed in accordance with the Helsinki Declaration of 1964, and its later amendments, and conformed with national regulations applicable during the study (Order SAS/3470/2009, of 16 December 2009, which published the Guidelines On Studies Post-authorization Of Observational Type For Medicinal Products For Human Use). The study had a cross-sectional design, and all patients provided written informed consent for participation in this study and publication of their clinical data for research purpose.
Data Availability
All data generated or analyzed during this study are included in this published article as supplementary information files.
Contributor Information
Ignacio Conget, Email: iconget@clinic.cat.
Miguel Ángel Mangas, Email: mangascruz@ono.com.
Cristóbal Morales, Email: cr.morales@hotmail.com.
Juan Caro, Email: juancaro84@gmail.com.
Margarita Giménez, Email: gimenez@clinic.ub.es.
Mireia Borrell, Email: Mireia.Borrell@sanofi.com.
Elías Delgado, Email: eliasdelga@gmail.com.
References
- 1.González Navarro R, Castro Jiménez JM, Gutiérrez Cuesta JL, Filippo Iriarte Gd, Osorio Dáguer MdR. Hemoglobina glicada como elemento de seguimiento y diagnóstico en las complicaciones de la diabetes mellitus en adultos mayores: Corporación Universitaria Rafael Núñez; 2020.
- 2.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the US: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 3.Mauricio D, Hramiak I. Second-generation insulin analogues—a review of recent real-world data and forthcoming head-to-head comparisons. Eur Endocrinol. 2018;14(Suppl1):2–9. doi: 10.17925/EE.2018.14supp1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heise T, Mathieu C. Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes. Diabetes Obes Metab. 2017;19(1):3–12. doi: 10.1111/dom.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergenstal RM, Bailey TS, Rodbard D, et al. Comparison of insulin glargine 300 units/ml and 100 units/ml in adults with type 1 diabetes: continuous glucose monitoring profiles and variability using morning or evening injections. Diabetes Care. 2017;40(4):554–560. doi: 10.2337/dc16-0684. [DOI] [PubMed] [Google Scholar]
- 6.Riddle M, Yki-Järvinen H, Bolli G, et al. One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the EDITION 1 12-month randomized trial, including 6-month extension. Diabetes Obes Metab. 2015;17(9):835–842. doi: 10.1111/dom.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893–902. doi: 10.1016/S2213-8587(16)30193-0. [DOI] [PubMed] [Google Scholar]
- 8.Bolli G, Riddle M, Bergenstal R, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naive people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3) Diabetes Obes Metab. 2015;17(4):386–394. doi: 10.1111/dom.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yki-Järvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2) Diabetes Care. 2014;37(12):3235–3243. doi: 10.2337/dc14-0990. [DOI] [PubMed] [Google Scholar]
- 10.Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units· mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units· mL−1. Diabetes Care. 2015;38(4):637–643. doi: 10.2337/dc14-0006. [DOI] [PubMed] [Google Scholar]
- 11.Ritzel R, Roussel R, Giaccari A, Vora J, Brulle-Wohlhueter C, Yki-Järvinen H. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/ml vs glargine 100 U/ml: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab. 2018;20(3):541–548. doi: 10.1111/dom.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (EDITION 4) Diabetes Care. 2015;38(12):2217–2225. doi: 10.2337/dc15-0249. [DOI] [PubMed] [Google Scholar]
- 13.Zhou FL, Ye F, Berhanu P, et al. Real-world evidence concerning clinical and economic outcomes of switching to insulin glargine 300 units/ml vs other basal insulins in patients with type 2 diabetes using basal insulin. Diabetes Obes Metab. 2018;20(5):1293–1297. doi: 10.1111/dom.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey TS, Wu J, Zhou FL, et al. Switching to insulin glargine 300 units/mL in real-world older patients with type 2 diabetes (DELIVER 3) Diabetes Obes Metab. 2019;21(11):2384–2393. doi: 10.1111/dom.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettus J, Roussel R, Zhou FL, et al. Rates of hypoglycemia predicted in patients with type 2 diabetes on insulin glargine 300 U/ml versus first-and second-generation basal insulin analogs: the real-world LIGHTNING study. Diabetes Ther. 2019;10(2):617–633. doi: 10.1007/s13300-019-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund P-O, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104–2114. doi: 10.1007/s11095-012-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tentolouris N, Knudsen ST, Lapolla A, Wolden ML, Haldrup S, Schultes B. Switching, “Real-World” diabetes patients to degludec from other basal insulins provides different clinical benefits according to their baseline glycemic control. Adv Ther. 2019;36(5):1201–1210. doi: 10.1007/s12325-019-00916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henao-Carrillo DC, Muñoz OM, Gómez AM, et al. Reduction of glycemic variability with Degludec insulin in patients with unstable diabetes. J Clin Transl Endocrinol. 2018;12:8–12. doi: 10.1016/j.jcte.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155–3162. doi: 10.1007/s00125-012-2708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371–378. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 21.Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367–1377. doi: 10.1016/S0140-6736(18)30297-6. [DOI] [PubMed] [Google Scholar]
- 22.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254–2263. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 23.Miura H, Sakaguchi K, Otowa-Suematsu N, et al. Effects of insulin degludec and insulin glargine U300 on glycaemic stability in individuals with type 1 diabetes: a multicentre, randomized controlled crossover study. Diabetes Obes Metab. 2020;22(12):2356–63. doi: 10.1111/dom.14161. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi Y, Sawa J, Sakuma N, Kumeda Y. Efficacy and safety of insulin glargine 300 U/mL vs insulin degludec in patients with type 2 diabetes: a randomized, open-label, cross-over study using continuous glucose monitoring profiles. J Diabetes Investig. 2019;10(2):343–351. doi: 10.1111/jdi.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomis R, Herrera-Pombo J, Calderón A, Rubio-Terrés C, Sarasa P. Validación del cuestionario “Diabetes treatment satisfaction questionnaire”(DTSQ) en la población española. PharmacoEconomics Spanish Research Articles. 2006;3(1):7–18. doi: 10.1007/BF03320906. [DOI] [Google Scholar]
- 27.Anderson RT, Skovlund SE, Marrero D, et al. Development and validation of the insulin treatment satisfaction questionnaire. Clin Ther. 2004;26(4):565–578. doi: 10.1016/S0149-2918(04)90059-8. [DOI] [PubMed] [Google Scholar]
- 28.Jansa M, Quiros C, Gimenez M, Vidal M, Galindo M, Conget I. Psychometric analysis of the Spanish and Catalan versions of a questionnaire for hypoglycemia awareness. Medicina Clínica (English Edition) 2015;144(10):440–444. doi: 10.1016/j.medcle.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laviola L, Porcellati F, Bruttomesso D, Larosa M, Rossi MC, Nicolucci A. Comparative effectiveness of switching from first-generation basal insulin to glargine 300 U/ml or degludec 100 U/ml in type 1 diabetes: the RESTORE-1 study. Diabetes Ther. 2021;12(2):509–525. doi: 10.1007/s13300-020-00982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey T, Pettus J, Roussel R, et al. Morning administration of 0.4 U/kg/day insulin glargine 300 U/mL provides less fluctuating 24-hour pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100 U/mL in type 1 diabetes. Diabetes Metab 2018;44(1):15–21. [DOI] [PubMed]
- 32.Heise T, Kaplan K, Haahr HL. Day-to-day and within-day variability in glucose-lowering effect between insulin degludec and insulin glargine (100 U/mL and 300 U/mL): a comparison across studies. J Diabetes Sci Technol. 2018;12(2):356–363. doi: 10.1177/1932296817731422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battelino T, Bosnyak Z, Danne T, et al. In Range: comparison of the second-generation basal insulin analogues glargine 300 U/mL and degludec 100 U/mL in persons with type 1 diabetes using continuous glucose monitoring—study design. Diabetes Ther. 2020;11(4):1017–27. doi: 10.1007/s13300-020-00781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Yang X, Huang J. Efficacy and safety of insulin degludec versus insulin glargine: a systematic review and meta-analysis of fifteen clinical trials. Int J Endocrinol. 2018;2018:8726046. doi: 10.1155/2018/8726046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Diabetes Care. 2018;41(10):2147–2154. doi: 10.2337/dc18-0559. [DOI] [PubMed] [Google Scholar]
- 36.San Laureano FC, Fernández-Ladreda MT, Millán AIJ, Calzado CG, Ortega MDCA. Insulin doses requirements in patients with type 1 diabetes using glargine U300 or degludec in routine clinical practice. J Investig Med. 2021;69(5):983–8. [DOI] [PMC free article] [PubMed]
- 37.Porcellati F, Lucidi P, Candeloro P, et al. Pharmacokinetics, pharmacodynamics, and modulation of hepatic glucose production with insulin glargine U300 and glargine U100 at steady state with individualized clinical doses in type 1 diabetes. Diabetes Care. 2019;42(1):85–92. doi: 10.2337/dc18-0706. [DOI] [PubMed] [Google Scholar]
- 38.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American DA. Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes. 2016;34(1):3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–1774. doi: 10.1007/s12325-018-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fadini GP, Feher M, Hansen TK, et al. Switching to degludec from other basal insulins is associated with reduced hypoglycemia rates: a prospective study. J Clin Endocrinol Metab. 2019;104(12):5977–5990. doi: 10.1210/jc.2019-01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article as supplementary information files.