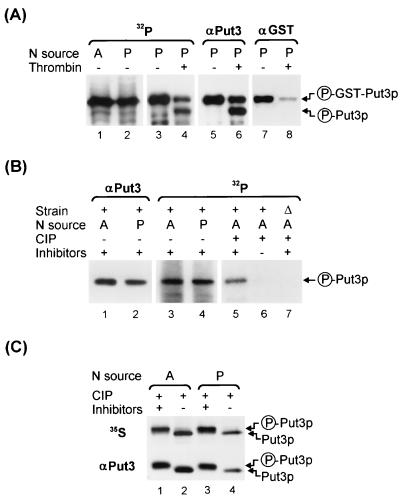
FIG. 1.
GST-Put3p and Put3p are phosphoproteins. (A) 32P labeling and IP of GST-Put3p. Extracts were made from cells of strain DB1000 carrying plasmid pHB3 (GST-PUT3) that were grown in a low-phosphate medium with ammonia (A; lane 1) or proline (P; lane 2) as the sole nitrogen source, labeled with 32P, and immunoprecipitated with mouse monoclonal anti-GST, as described in Materials and Methods. The immunoprecipitated GST-Put3p from the proline culture (lane 3) was incompletely digested with thrombin to release full-length Put3p (lane 4). After the signal decayed, the membrane was probed with anti-Put3p (lanes 5 and 6) and then reprobed with anti-GST antibodies (lanes 7 and 8) for detection of GST-Put3p and the cleaved Put3p. The proteins were resolved on a 12.5% (12.57% total acrylamide concentration [T], 0.5% cross-linker concentration [C]) polyacrylamide gel and transferred to a PVDF membrane. Circled P, phosphorylated form. (B) 32P labeling and IP of Put3p. Extracts were made from cells of strain DB1000 carrying plasmid pDB37 (PUT3) grown in a low-phosphate medium with ammonia (lane 3) or proline (lane 4) as the sole nitrogen source or carrying YEp24 grown in a low-phosphate medium with ammonia (lane 7), labeled with 32P, and immunoprecipitated with polyclonal anti-Put3p, as described in Materials and Methods. The proteins were resolved on a 7.5% (7.65% T, 2% C) polyacrylamide gel and transferred to a PVDF membrane. After the signal decayed, the membrane was probed with anti-Put3p antibody (lanes 1 and 2). The 32P-labeled immunoprecipitates from the ammonia cultures were treated with CIP in the presence (+) or absence (−) of phosphatase inhibitors (lanes 5 and 6, wild type; lane 7, put3Δ). (C) 35S labeling of Put3p. Extracts of 35S-labeled DB1000 carrying plasmid pDB37 grown on ammonia- or proline-containing medium were immunoprecipitated with anti-Put3p antibody (lanes 1 to 4, upper section) and treated with CIP in the presence (lanes 1 and 3) or absence (lanes 2 and 4) of phosphatase inhibitors. The proteins were resolved on a 7.5% (7.65% T, 2% C) polyacrylamide gel and transferred to a PVDF membrane. After the signal decayed, the membrane was probed with anti-Put3p antibody (lanes 1 to 4, lower section). These gels were run under conditions that do not resolve isoforms of Put3p.