Abstract
Background
Advanced glycation end-products, indicated by skin autofluorescence (SAF) levels, could be prognostic predictors of all-cause and cardiovascular mortality in patients with diabetes mellitus (DM) and renal disease. However, the clinical usefulness of SAF levels in patients with heart failure (HF) who underwent cardiac rehabilitation (CR) remains unclear. This study aimed to investigate the associations between SAF and MACE risk in patients with HF who underwent CR.
Methods
This study enrolled 204 consecutive patients with HF who had undergone CR at our university hospital between November 2015 and October 2017. Clinical characteristics and anthropometric data were collected at the beginning of CR. SAF levels were noninvasively measured with an autofluorescence reader. Major adverse cardiovascular event (MACE) was a composite of all-cause mortality and unplanned hospitalization for HF. Follow-up data concerning primary endpoints were collected until November 2017.
Results
Patients’ mean age was 68.1 years, and 61% were male. Patients were divided into two groups according to the median SAF levels (High and Low SAF groups). Patients in the High SAF group were significantly older, had a higher prevalence of chronic kidney disease, and more frequently had history of coronary artery bypass surgery; however, there were no significant between-group differences in sex, prevalence of DM, left ventricular ejection fraction, and physical function. During a mean follow-up period of 590 days, 18 patients had all-cause mortality and 36 were hospitalized for HF. Kaplan–Meier analysis showed that patients in the high SAF group had a higher incidence of MACE (log-rank P < 0.05). After adjusting for confounding factors, Cox regression multivariate analysis revealed that SAF levels were independently associated with the incidence of MACE (odds ratio, 1.86; 95% confidence interval, 1.08–3.12; P = 0.03).
Conclusion
SAF levels were significantly associated with the incidence of MACE in patients with HF and may be useful for risk stratification in patients with HF who underwent CR.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-021-01398-0.
Introduction
Heart failure (HF) is closely associated with diabetes mellitus (DM), and patients with HF with DM have worse outcomes than those without DM [1]. The development of vascular complications associated with DM involves multiple dysfunction pathways, and among these damage pathways, advanced glycation end-products (AGEs) accumulation has gained particular attention [2]. Reducing sugars, such as glucose, are non-enzymatically bound to the amino groups of proteins, pass through Amadori compounds, and, after multiple reactions, change to irreversible AGEs [3]. AGEs rapidly accumulate in whole body tissues not only with aging but also under conditions such as hyperglycemia and chronic inflammation [4]. Moreover, AGEs form cross-links with vascular and muscle proteins, leading to physiological dysfunction in multiple organ systems [4]. AGEs that accumulate in the skin can be measured by a noninvasive method using the skin autofluorescence (SAF) value [5]. Recently, SAF level is reported to be useful as a prognostic marker for high-risk patients, such as patients with DM and chronic kidney disease (CKD) [6]. However, the relationship between SAF levels and clinical outcomes in patients with HF undergoing cardiac rehabilitation (CR) remains unclear. Thus, this study aimed to investigate the relationship between SAF levels and clinical prognosis in patients with chronic HF who have undergone CR.
Methods
Study population
This was a retrospective observational study of 249 consecutive patients with HF who participated in phase II CR at our university hospital between November 2015 and September 2017. HF was defined according to the 2013 ACCF/AHA Guideline for the Management of HF [7]. Patients categorized as Stage B and C according to the ACCF/AHA classification that were determined to have indications for phase II CR by attending physician participated in the study. Stage D patients who could not undergo phase II CR were excluded. Of them, 45 patients who lacked baseline SAF data were excluded. The final study population consisted of 204 patients (Fig. 1). Written informed consent was provided by all patients before participation. The study protocol was approved by the ethics committee of our institution and conducted in accordance with the Declaration of Helsinki.
Fig. 1.
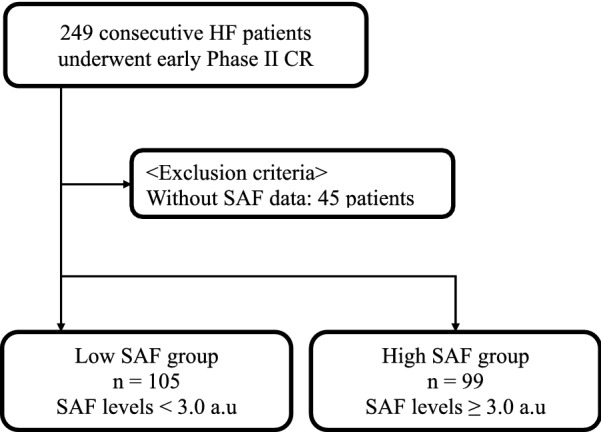
Study flowchart of all subjects. HF heart failure, CR cardiac rehabilitation, SAF skin autofluorescence
Data collection and measurements
Age, sex, smoking history, comorbidities, and medical history were obtained from patient medical records. Blood samples were collected in the early morning after overnight fasting. A diagnosis of DM was defined by hemoglobin A1c level ≥ 6.5 or receiving treatment for DM. CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, calculated using the renal disease equation with the Japanese coefficient using baseline serum creatinine level and modification to diet [8]. Body composition, grip strength, and SAF level were assessed at the beginning of CR. Anthropometric parameters, including body fat percentage, lean body weight, and muscle mass, were measured by bioelectrical impedance analysis (TANITA, MC-780A, Tokyo, Japan), as previously described [9]. Grip strength test was conducted in both hands in a standing position; the higher grip strength value was used. Exercise capacity was assessed using the cardiopulmonary exercise test (CPX) on a cycle ergometer (Strength ergo 8®) with an expiratory gas analysis machine (AE-310S®) using a ramp protocol to measure peak oxygen uptake (peakVO2) at the beginning of the CR.
SAF
The SAF levels were measured by AGE Reader (DiagnOptics Technologies B.V., Groningen, The Netherlands) [5]. Briefly, the AGE Reader can noninvasively evaluate the accumulation of AGEs in the skin as the levels of fluorescence with excitation light [5]. SAF levels were calculated as the ratio of the average light intensity in the 420–600 nm wavelength range and average excitation light intensity in the 300–420 nm range. In the epithelium and dermis of the skin, AGEs are known to bind to collagen and elastin and accumulate [10]. A previous study on healthy subjects and those with DM showed that SAF levels assessed by the AGE Reader correlated well with the accumulation of AGEs, such as pentosidine and carboxymethyl-lysine, assessed by skin biopsy [11]. Therefore, SAF reflects mainly the accumulation of AGEs in the epithelium and dermis of the skin. Report has shown an intra-individual error percentage of approximately 5% for repeat skin AF measurements taken within a day [5]. Therefore, in the present study, SAF levels were measured only once from the inside of either forearm with patients in the sitting position.
Primary endpoints
The primary outcome was major adverse cardiac events, defined as a composite of all-cause mortality and unplanned HF-related hospitalization. Mortality and hospitalization data were collected from the medical records of patients who died or were treated at our institution.
Statistical analysis
Continuous variables were presented as mean ± standard deviation, and categorical variables were expressed as counts and percentages. Comparisons between groups were performed using Welch’s t-test for continuous variables and chi-squared test for categorical variables. Unadjusted cumulative event rate for the primary endpoint was estimated using the Kaplan–Meier method and compared between groups using the log-rank test. The cut-off value was defined using the median of the SAF level (3.0 AU). Univariate and multivariate Cox regression analyses were performed to identify the predictor of the primary endpoints. Hazard ratios and 95% confidence intervals (CIs) were also calculated. SAF, age, BMI, history of CABG, hemoglobin, albumin, eGFR, HbA1c, triglyceride, LDL-cholesterol, HDL-cholesterol, BNP, aspirin, statin, β-blockers, and oral hypoglycemic agent were selected as cofounding factors. Hazard ratios for continuous variables are expressed per SD change. Differences were considered significant at a P-value < 0.05. JMP version 12.0 (SAS Institute, Cary, NC, USA) was used to perform statistical analyses.
Results
The mean patient age was 68 ± 15 years, and 125 patients (61.2%) were male. The patients were followed until December 2018. The mean follow-up duration was 590 ± 293 days.
Figure 1 presents the patient flowchart and exclusion criteria. Additional file 1: Figure S1 shows the distribution of SAF levels. The mean and median SAF levels were 3.0 ± 0.7 AU (mean ± SD) and 3.0 AU, respectively (range, 1.4–5.4 AU).
Based on the median SAF level (3.0 AU), patients were divided into two groups (High SAF and Low SAF). The High and Low SAF groups comprised 105 and 99 participants, respectively. Table 1 shows the clinical characteristics of the High and Low SAF groups. The two groups did not show significant difference in sex ratio. The High SAF group was significantly older and had a higher prevalence of CKD and history of coronary artery bypass graft. Hemoglobin, albumin, and eGFR levels were significantly lower, and fasting blood sugar and brain natriuretic peptide (BNP) levels were higher in the High SAF group. Cardiac function and grip strength were similar between the two groups. Patients in the High SAF group were more frequently prescribed with aspirin, statin, β-blockers, and oral hypoglycemic agent. CPX was performed in 55 and 33 cases in the Low and High SAF groups, respectively. The High SAF group had significantly lower exercise capacity compared to the Low SAF group. Additionally, we compared between DM and non-DM groups. Details are presented in Additional file 3: Table S2.
Table 1.
Patient characteristics
| Low SAF (n = 99) | High SAF (n = 105) | P-value | |
|---|---|---|---|
| Age | 61.5 ± 16.0 | 74.3 ± 10.5 | < 0.01 |
| Male (%) | 60 (60.6) | 65 (61.9) | 0.84 |
| BMI | 23.0 ± 4.0 | 23.1 ± 4.1 | 0.74 |
| Hypertension (%) | 50 (50.5) | 60 (57.1) | 0.34 |
| Diabetes mellitus (%) | 35 (35.4) | 39 (37.1) | 0.79 |
| Dyslipidemia (%) | 39 (39.4) | 43 (41.0) | 0.82 |
| Chronic kidney disease (%) | 45 (45.9) | 65 (62.5) | 0.02 |
| Current smoking (%) | 14 (14.3) | 10 (9.6) | 0.30 |
| COPD (%) | 1 (1.0) | 5 (4.8) | 0.11 |
| History of MI (%) | 12 (12.1) | 22 (21.0) | 0.08 |
| History of PCI (%) | 15 (15.2) | 26 (24.8) | 0.08 |
| History of CABG (%) | 6 (6.1) | 24 (22.9) | < 0.01 |
| History of valvular surgery (%) | 10 (10.1) | 13 (12.4) | 0.60 |
| History of CHF (%) | 49 (49.5) | 56 (53.9) | 0.53 |
| Valvular disease (%) | |||
| Aortic valve stenosis | 11 (11.1) | 22 (21.0) | 0.06 |
| Aortic valve regurgitation | 2 (2.0) | 3 (2.9) | 0.70 |
| Mitral valve stenosis | 1 (1.0) | 1 (1.0) | 0.96 |
| Mitral valve regurgitation | 15 (15.2) | 27 (25.7) | 0.06 |
| Tricuspid valve regurgitation | 15 (15.2) | 13 (12.4) | 0.57 |
| Atrial fibrillation (%) | 30 (30.3) | 36 (34.3) | 0.54 |
| Dilated cardiomyopathy (%) | 12 (12.1) | 8 (7.6) | 0.27 |
| Echocardiography | |||
| LVEF (%) | 49 ± 20 | 51 ± 18 | 0.46 |
| E/e′ | 19.1 ± 11.0 | 20.0 ± 11.7 | 0.77 |
| Laboratory data | |||
| Hemoglobin, g/dL | 13.2 ± 2.3 | 12.2 ± 2.1 | < 0.01 |
| Albumin, g/dL | 3.7 ± 0.5 | 3.6 ± 0.5 | 0.04 |
| HbA1c (%) | 6.0 ± 0.7 | 6.1 ± 0.9 | 0.36 |
| Fasting blood sugar, mg/dL | 97 ± 21 | 106 ± 30 | 0.02 |
| Total cholesterol, mg/dL | 167 ± 33 | 165 ± 38 | 0.69 |
| LDL-cholesterol, mg/dL | 98 ± 27 | 96 ± 32 | 0.76 |
| HDL-cholesterol, mg/dL | 46 ± 14 | 46 ± 12 | 0.85 |
| Triglyceride, mg/dL | 109 ± 54 | 104 ± 46 | 0.53 |
| eGFR, mL/min/1.73 m2 | 66.5 ± 27.5 | 51.9 ± 25.0 | < 0.01 |
| BNP, pg/dL | 325.4 ± 333.4 | 516.4 ± 862.7 | 0.04 |
| Medication | |||
| Aspirin (%) | 27 (27.3) | 52 (50.0) | < 0.01 |
| ACE-I/ARB (%) | 67 (67.7) | 72 (69.2) | 0.81 |
| Statin (%) | 44 (44.4) | 63 (60.6) | 0.02 |
| β-Blocker (%) | 70 (70.7) | 87 (83.7) | 0.03 |
| Ca antagonist (%) | 27 (27.3) | 37 (35.6) | 0.20 |
| Loop diuretics (%) | 63 (63.6) | 75 (72.1) | 0.19 |
| Oral hypoglycemic agent (%) | 10 (10.1) | 22 (21.0) | 0.03 |
| Insulin (%) | 4 (4.0) | 9 (8.6) | 0.19 |
| Anthropometric data and grip strength | |||
| Body fat percentage (%) | 23.9 ± 7.8 | 24.9 ± 10.7 | 0.48 |
| Lean body weight (kg) | 46.7 ± 11.3 | 44.0 ± 9.9 | 0.11 |
| Grip strength (kg) | 28.0 ± 10.6 | 25.5 ± 8.1 | 0.23 |
Endpoints
For the entire duration of follow-up, 18 patients in the Low SAF groups and 36 patients in the High SAF groups had a primary event (Additional file 2: Table S1). Kaplan–Meier analysis was performed to estimate the unadjusted event-free rate of primary endpoints. Event-free survival rate was significantly lower in the High SAF group than in the Low SAF group (P = 0.01, Fig. 2). Univariate Cox regression analyses revealed that SAF level was associated with the primary composite endpoint (odds ratio, 2.00; 95% CI 1.41–2.78; P < 0.01, Table 2). After adjustment for confounding variables, SAF level was significantly associated with long-term primary composite endpoint (odds ratio, 1.86; 95% CI 1.08–3.12; P = 0.03, Table 2).
Fig. 2.
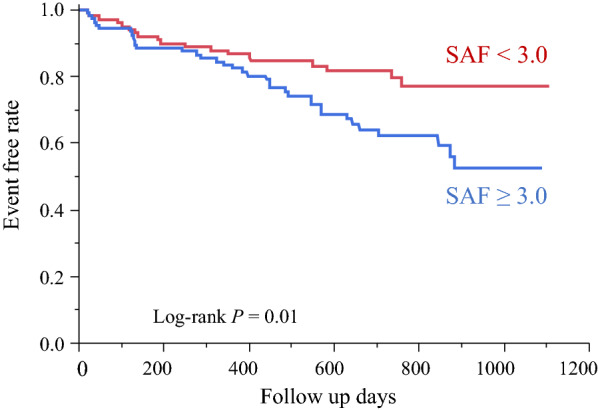
Kaplan–Meier survival curves. SAF skin autofluorescence
Table 2.
Univariate and multivariate Cox regression analyses of MACE
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| SAF | 2.00 | 1.41–2.78 | < 0.01 | 1.86 | 1.08–3.12 | 0.03 |
| Age | 1.03 | 1.01–1.05 | < 0.01 | 1.01 | 0.98–1.04 | 0.67 |
| BMI | 0.90 | 0.84–0.97 | < 0.01 | 0.88 | 0.80–0.96 | < 0.01 |
| History of CABG | 2.24 | 1.17–4.01 | 0.02 | 1.49 | 0.63–3.38 | 0.35 |
| Hemoglobin | 0.77 | 0.67–0.87 | < 0.01 | 0.95 | 0.78–1.15 | 0.58 |
| Albumin | 0.54 | 0.30–0.96 | 0.03 | 1.93 | 0.86–4.30 | 0.10 |
| eGFR | 0.97 | 0.96–0.98 | < 0.01 | 0.98 | 0.97–0.99 | 0.01 |
| HbA1c | 0.94 | 0.65–1.30 | 0.73 | 1.20 | 0.77–1.79 | 0.41 |
| Triglyceride | 0.99 | 0.99–1.00- | 0.051 | 0.99 | 0.99–1.00 | 0.15 |
| LDL-cholesterol | 0.99 | 0.98–0.99 | 0.01 | 0.98 | 0.97–0.99 | 0.03 |
| HDL-cholesterol | 1.00 | 0.98–1.02 | 0.83 | 1.00 | 0.98–1.03 | 0.77 |
| BNP | 1.006 | 1.00–1.00 | < 0.01 | 1.00 | 0.99–1.00 | 0.27 |
| Aspirin | 1.22 | 0.70–2.09 | 0.47 | 0.54 | 0.27–1.06 | 0.07 |
| Statin, | 1.18 | 0.69–2.05 | 0.55 | 1.24 | 0.57–2.69 | 0.59 |
| β-Blockers | 2.17 | 1.05–5.28 | 0.04 | 2.00 | 0.82–5.74 | 0.13 |
| Oral hypoglycemic agent | 1.09 | 0.50–2.11 | 0.83 | 0.84 | 0.30–2.10 | 0.72 |
MACE major adverse cardiovascular event, CI confidence interval, SAF skin autofluorescence, BMI body mass index, CABG coronary artery bypass graft, eGFR estimated glomerular filtration rate, HbA1c hemoglobin A1c, LDL low-density lipoprotein, HDL high-density lipoprotein, BNP brain natriuretic peptide
Discussion
The present study demonstrated that higher SAF levels are significantly and independently associated with combined endpoint (all-cause death and unplanned HF-related hospitalization). To the best of our knowledge, this is the first study to demonstrate an association between SAF levels and adverse outcomes in patients with cardiovascular disease (CVD) who underwent CR.
In large clinical trials that analyze HbA1c levels, HbA1c was controlled at a lower level in the intensive care group than in the conventional treatment group, but macrovascular complications could not be significantly suppressed [12–14]. However, in the treatment of DM, strict glycemic control from the early stage of onset is extremely important. Previous studies have shown that, after long-term follow-up, adverse events, including death, were suppressed by the early intensive care group even after the intensive and conventional treatment groups eventually showed the same level of glycemic control [15, 16]. It is possible that early glycemic control may have long-term beneficial effects, so called “metabolic memory” [17]. AGEs may be used to explain the legacy effect because AGEs are metabolized very slowly and poorly degraded and remain in various types of diabetic tissue for a long period [4]. AGEs promote the production of intracellular oxidative stress after recognition by a receptor for AGEs (RAGE) and then activate nuclear factor κB and induce the secretion of multiple cytokines and growth factors and upregulation of adhesion factor [2]. The increase in oxidative stress caused by the AGE–RAGE system inactivates nitric oxide and promotes the inflammatory reaction and thrombotic tendency, leading to the onset and progression of arteriosclerosis [18, 19]. Accumulation of AGEs is associated with cardiovascular dysfunction [20]. In fact, the higher the concentration of AGEs in the blood, the higher the severity of HF, and AGEs are a poor prognostic factor in patients with HF [21, 22]. Moreover, AGEs have been associated with liver disease, lung disease, and malignant tumors [23–25].
Previous studies have reported that SAF levels were associated with vascular and multiorgan dysfunction [26] and cardiovascular adverse event in both high-risk patients and general populations [27–30]. Cavero-Redondo et al. reported that higher SAF levels were significantly associated with twofold risk of cardiovascular and all-cause mortality in high-risk patients [27]. In a study of patients with type 2 diabetes, Henderikus E Boersma reported that SAF was significantly associated with the development of new cardiovascular disease and death [31]. Waateringe et al. reported that, for every unit of SAF increase, the risk of developing CVD increases threefold and the risk of death increases fivefold in a study with 72,880 participants [30]. Additionally, a study on patients with HF with preserved ejection fraction found that higher SAF levels (SAF > 2.9 AU) were associated with first hospitalization for HF [32]. Moreover, our teams have recently reported that exercise capacity, a strong predictor in patients with CVD, was significantly lower in patients with higher SAF levels regardless of their DM status and that SAF levels were independently associated with exercise intolerance in patients with CVD [9]. The present study is the first to report that SAF levels are significantly associated with adverse outcomes in patients with HF who underwent CR. The clear cutoff value of SAF for diagnosis of risk remains unclear. The study population and number of events were relatively small; therefore, the cutoff value was defined using the median SAF level. However, even in the previous study [28], the median value was 2.69 AU (interquartile range, 2.26–3.19 AU), which is close to our data, and it seems to be appropriate. The present study revealed that eGFR levels were associated with primary endpoints. Previous studies had reported that lower eGFR was independently associated with death from any cause and HF hospitalization in patients with HF [33]. Moreover, other studies investigating SAF as a prognostic factor for cardiovascular events and death in patients with earlier stages of CKD revealed that both SAF and higher eGFR were independently associated with a decreased risk of cardiovascular events and death [34]. Our findings are consistent with those of previous reports. Univariate Cox regression analysis in the present study also found hemoglobin level to be associated with an increased risk of MACE. This vicious cycle of HF, renal failure, and anemia is known as cardiorenal anemia syndrome [35]. The relationship among the three factors causes vascular endothelial damage through overproduction of reactive oxygen species and impaired fluid regulation; furthermore, the progression of arteriosclerosis and increase in cardiovascular and renal load due to extracellular fluid retention are the main pathological conditions of this cycle [35]. We also identified BMI as a significant factor, with a 12% decrease in MACE for each 1-kg/m2 increase in BMI. Previous studies have reported that patients with HF who had higher BMI exhibited good prognosis, a phenomenon recognized as the “obesity paradox” [36]. Given that HF is a catabolic state, obese patients may exhibit better prognosis considering that they have more metabolic reserve [37, 38], allowing them to store more energy, which could be favorable for heart failure. Tumor necrosis factor (TNF)-α level has been known to increase in HF, thereby resulting in worsening of HF. Adipocytes produce antibodies that neutralize TNF-α, which acts as an anti-inflammatory [39]. Increased lean body mass was associated with better physical function, suggesting that skeletal muscle metabolism plays a central role in the exercise tolerance of patients with HF [40]. These facts may be associated with better clinical outcomes in such patients.
Limitations
This study has several limitations. First, this study was conducted in a single center, and sample size was relatively small. Studies with a larger sample size will be more effective in evaluating the association between SAF levels and prognosis in patients with CVD who underwent CR. Second, we could not investigate other therapeutic interventions during the observation period. Third, we could not track the changes in SAF level over time. Fourth, the implementation rate of CPX was low. Fifth, SAF represents not only the fluorescence value generated by AGE of the skin but also that generated by other fluorophores, such as keratin [41]. Skin color and the use of skin creams can also affect SAF [42, 43]. However, only Japanese patients had been included herein, none of whom seemed to demonstrate excessive skin cream use. Although our data showed an association between SAF levels and adverse outcomes in patients with CVD, further intervention studies aimed at reducing AGE accumulation, as in previous studies [44], need to be conducted.
Conclusion
SAF levels in patients with HF undergoing CR may be a predictor of all-cause mortality and HF hospitalization independent of multiple factors, and measurement of SAF level may provide useful information in patients undergoing CR.
Supplementary Information
Additional file 1: Figure S1. Distribution of SAF levels.
Additional file 2: Table S1. Occurrence of composite endpoints.
Additional file 3: Table S2. Comparison of clinical characteristics between DM and non-DM groups.
Acknowledgements
The authors wish to thank all study participants and members of the data collection group in Cardiovascular Rehabilitation and Fitness.
Abbreviations
- AGEs
Advanced glycation end-products
- BMI
Body mass index
- BNP
B-type natriuretic peptide
- CI
Confidence interval
- CKD
Chronic kidney disease
- CR
Cardiac rehabilitation
- CVD
Cardiovascular disease
- DM
Diabetes mellitus
- eGFR
Estimated glomerular filtration rate
- HbA1c
Hemoglobin A1c
- HDL
High density lipoprotein
- HF
Heart failure
- LDL
Low density lipoprotein
- MACE
Major adverse cardiovascular event
- MI
Myocardial infarction
- SAF
Skin autofluorescence
- TG
Triglyceride
Authors’ contributions
MK, MY, KS, TT, HD, and TM4 contributed to the conception and design of the work. AA2, MS, and TM3 contributed to the conception of the work. MK, KS, and TM2 contributed to the acquisition, analysis, and interpretation of data. KF1, AH, AA1, AS, and TY contributed to the acquisition of data. TM1, TA, SO, and KF2 contributed to the interpretation of data. MK drafted the manuscript. All authors critically revised the manuscript. All agree to be accountable for all aspects of work ensuring integrity and accuracy. All authors read and approved the final manuscript.
Funding
This study was supported in part by JSPS KAKENHI Grant Number 17K01470 and the High Technology Research Center Grant from the Ministry of Education, Culture, Science, and Technology, Japan.
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to data protection regulations.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of our institution and conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3(2):136–145. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Kovacic JC, Castellano JM, Farkouh ME, Fuster V. The relationships between cardiovascular disease and diabetes: focus on pathogenesis. Endocrinol Metab Clin North Am. 2014;43(1):41–57. doi: 10.1016/j.ecl.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63(4):582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Yamagishi S. Potential clinical utility of advanced glycation end product cross-link breakers in age- and diabetes-associated disorders. Rejuvenation Res. 2012;15(6):564–572. doi: 10.1089/rej.2012.1335. [DOI] [PubMed] [Google Scholar]
- 5.Meerwaldt R, Graaff R, Oomen PHN, Links TP, Jager JJ, Alderson NL, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47(7):1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 6.Cavero-Redondo I, Soriano-Cano A, Alvarez-Bueno C, Cunha PG, Martinez-Hortelano JA, Garrido-Miguel M, et al. Skin autofluorescence-indicated advanced glycation end products as predictors of cardiovascular and all-cause mortality in high-risk subjects: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(18):e009833. doi: 10.1161/JAHA.118.009833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Kunimoto M, Shimada K, Yokoyama M, Matsubara T, Aikawa T, Ouchi S, et al. Association between the tissue accumulation of advanced glycation end products and exercise capacity in cardiac rehabilitation patients. BMC Cardiovasc Disord. 2020;20(1):195. doi: 10.1186/s12872-020-01484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4(3):259–270. doi: 10.4161/derm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, et al. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2654–2659. doi: 10.2337/dc05-2173. [DOI] [PubMed] [Google Scholar]
- 12.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 13.Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 15.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi SI. Role of advanced glycation endproduct (AGE)-receptor for advanced glycation endproduct (RAGE) axis in cardiovascular disease and its therapeutic intervention. Circ J. 2019;83(9):1822–1828. doi: 10.1253/circj.CJ-19-0618. [DOI] [PubMed] [Google Scholar]
- 18.Yamagishi S, Ueda S, Matsui T, Nakamura K, Imaizumi T, Takeuchi M, et al. Pigment epithelium-derived factor (PEDF) prevents advanced glycation end products (AGEs)-elicited endothelial nitric oxide synthase (eNOS) reduction through its anti-oxidative properties. Protein Pept Lett. 2007;14(8):832–835. doi: 10.2174/092986607781483705. [DOI] [PubMed] [Google Scholar]
- 19.Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, Yamagishi S, et al. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 2008;13(11):1159–1170. doi: 10.1111/j.1365-2443.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 20.Hartog JW, Voors AA, Bakker SJ, Smit AJ, van Veldhuisen DJ. Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. Eur J Heart Fail. 2007;9(12):1146–1155. doi: 10.1016/j.ejheart.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Koyama Y, Takeishi Y, Arimoto T, Niizeki T, Shishido T, Takahashi H, et al. High serum level of pentosidine, an advanced glycation end product (AGE), is a risk factor of patients with heart failure. J Card Fail. 2007;13(3):199–206. doi: 10.1016/j.cardfail.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Willemsen S, Hartog JW, van Veldhuisen DJ, van der Meer P, Roze JF, Jaarsma T, et al. The role of advanced glycation end-products and their receptor on outcome in heart failure patients with preserved and reduced ejection fraction. Am Heart J. 2012;164(5):742–9.e3. doi: 10.1016/j.ahj.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Sakasai-Sakai A, Takata T, Takino JI, Takeuchi M. The relevance of toxic AGEs (TAGE) cytotoxicity to NASH pathogenesis: a mini-review. Nutrients. 2019;11(2):462. doi: 10.3390/nu11020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takino J, Yamagishi S, Takeuchi M. Cancer malignancy is enhanced by glyceraldehyde-derived advanced glycation end-products. J Oncol. 2010;2010:739852. doi: 10.1155/2010/739852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Modern Pathol. 2006;19(11):1437–1445. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- 26.Yamagishi S, Fukami K, Matsui T. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: a novel marker of vascular complications in high-risk patients for cardiovascular disease. Int J Cardiol. 2015;185:263–268. doi: 10.1016/j.ijcard.2015.03.167. [DOI] [PubMed] [Google Scholar]
- 27.Cavero-Redondo I, Soriano-Cano A, Álvarez-Bueno C, Cunha PG, Martínez-Hortelano JA, Garrido-Miguel M, et al. Skin autofluorescence-indicated advanced glycation end products as predictors of cardiovascular and all-cause mortality in high-risk subjects: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(18):e009833. doi: 10.1161/JAHA.118.009833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutgers HL, Gerrits EG, Graaff R, Links TP, Sluiter WJ, Gans RO, et al. Skin autofluorescence provides additional information to the UK prospective diabetes study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia. 2009;52(5):789–797. doi: 10.1007/s00125-009-1308-9. [DOI] [PubMed] [Google Scholar]
- 29.Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, et al. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care. 2007;30(1):107–112. doi: 10.2337/dc06-1391. [DOI] [PubMed] [Google Scholar]
- 30.van Waateringe RP, Fokkens BT, Slagter SN, van der Klauw MM, van Vliet-Ostaptchouk JV, Graaff R, et al. Skin autofluorescence predicts incident type 2 diabetes, cardiovascular disease and mortality in the general population. Diabetologia. 2019;62(2):269–280. doi: 10.1007/s00125-018-4769-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boersma HE, van Waateringe RP, van der Klauw MM, Graaff R, Paterson AD, Smit AJ, et al. Skin autofluorescence predicts new cardiovascular disease and mortality in people with type 2 diabetes. BMC Endocr Disord. 2021;21(1):14. doi: 10.1186/s12902-020-00676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hitsumoto T. Skin autofluorescence as a predictor of first heart failure hospitalization in patients with heart failure with preserved ejection fraction. Cardiol Res. 2020;11(4):247–255. doi: 10.14740/cr1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith DH, Thorp ML, Gurwitz JH, McManus DD, Goldberg RJ, Allen LA, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the cardiovascular research network PRESERVE study. Circ Cardiovasc Qual Outcomes. 2013;6(3):333–342. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shardlow A, McIntyre NJ, Kolhe NV, Nellums LB, Fluck RJ, McIntyre CW, et al. The association of skin autofluorescence with cardiovascular events and all-cause mortality in persons with chronic kidney disease stage 3: a prospective cohort study. PLoS Med. 2020;17(7):e1003163. doi: 10.1371/journal.pmed.1003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverberg DS, Wexler D, Iaina A, Steinbruch S, Wollman Y, Schwartz D. Anemia, chronic renal disease and congestive heart failure—the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol. 2006;38(2):295–310. doi: 10.1007/s11255-006-0064-8. [DOI] [PubMed] [Google Scholar]
- 36.Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115(10):1428–1434. doi: 10.1016/j.amjcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Berry C, Clark AL. Catabolism in chronic heart failure. Eur Heart J. 2000;21(7):521–532. doi: 10.1053/euhj.1999.1882. [DOI] [PubMed] [Google Scholar]
- 38.Imbeault P, Tremblay A, Simoneau JA, Joanisse DR. Weight loss-induced rise in plasma pollutant is associated with reduced skeletal muscle oxidative capacity. Am J Physiol Endocrinol Metab. 2002;282(3):E574–E579. doi: 10.1152/ajpendo.00394.2001. [DOI] [PubMed] [Google Scholar]
- 39.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277(6):E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 40.Okita K, Kinugawa S, Tsutsui H. Exercise intolerance in chronic heart failure–skeletal muscle dysfunction and potential therapies. Circ J. 2013;77(2):293–300. doi: 10.1253/circj.CJ-12-1235. [DOI] [PubMed] [Google Scholar]
- 41.Koetsier M, Nur E, Chunmao H, Lutgers HL, Links TP, Smit AJ, et al. Skin color independent assessment of aging using skin autofluorescence. Opt Express. 2010;18(14):14416–14429. doi: 10.1364/OE.18.014416. [DOI] [PubMed] [Google Scholar]
- 42.Mulder DJ, Water TV, Lutgers HL, Graaff R, Gans RO, Zijlstra F, et al. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: an overview of current clinical studies, evidence, and limitations. Diabetes Technol Ther. 2006;8(5):523–535. doi: 10.1089/dia.2006.8.523. [DOI] [PubMed] [Google Scholar]
- 43.Noordzij MJ, Lefrandt JD, Graaff R, Smit AJ. Dermal factors influencing measurement of skin autofluorescence. Diabetes Technol Ther. 2011;13(2):165–170. doi: 10.1089/dia.2010.0123. [DOI] [PubMed] [Google Scholar]
- 44.Hartog JW, Willemsen S, van Veldhuisen DJ, Posma JL, van Wijk LM, Hummel YM, et al. Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure. Eur J Heart Fail. 2011;13(8):899–908. doi: 10.1093/eurjhf/hfr067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Distribution of SAF levels.
Additional file 2: Table S1. Occurrence of composite endpoints.
Additional file 3: Table S2. Comparison of clinical characteristics between DM and non-DM groups.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to data protection regulations.


