Abstract
Introduction
Advanced or metastatic soft tissue sarcoma (a/mSTS) is associated with a dismal prognosis. Patient counseling on treatment aggressiveness is pivotal to avoid over- or undertreatment. Recently, evaluation of body composition markers like the skeletal muscle index (SMI) became focus of interest in a variety of cancers. This study focuses on the prognostic impact of SMI in a/mSTS, retrospectively.
Methods
181 a/mSTS patients were identified, 89 were eligible due to prespecified criteria for SMI assessment. Baseline CT-Scans were analyzed using an institutional software solution. Sarcopenia defining cut-off values for the SMI were established by optimal fitting method. Primary end point was overall survival (OS) and secondary endpoints were progression free survival (PFS), disease control rate (DCR), overall response rate (ORR). Descriptive statistics as well as Kaplan Meier- and Cox regression analyses were administered.
Results
28/89 a/mSTS patients showed sarcopenia. Sarcopenic patients were significantly older, generally tended to receive less multimodal therapies (62 vs. 57 years, P = 0.025; respectively median 2.5 vs. 4, P = 0.132) and showed a significantly lower median OS (4 months [95%CI 1.9–6.0] vs. 16 months [95%CI 8.8–23.2], Log-rank P = 0.002). Sarcopenia was identified as independent prognostic parameter of impaired OS (HR 2.40 [95%-CI 1.4–4.0], P < 0.001). Moreover, DCR of first palliative medical treatment was superior in non-sarcopenic patients (49.2% vs. 25%, P = 0.032).
Conclusion
This study identifies sarcopenia as a prognostic parameter in a/mSTS. Further on, the data suggest that sarcopenia shows a trend of being associated with first line therapy response. SMI is a promising prognostic parameter, which needs further validation.
Keywords: Sarcopenia, Soft tissue sarcoma, Smi, Skeletal muscle index, Survival, Body composition
Introduction
Despite improvements in surgical techniques, pathological understanding and medical treatment, patients with advanced or metastatic soft tissue sarcomas (a/mSTS) are mostly considered incurable [1]. Recent studies report a median overall survival (OS) of 10–18 months, and the 5-year survival rate for metastatic sarcoma is a mere 16% [2–4]. Also, despite several new tested drugs, as well as several discussed treatment strategies, during the past decade, no substantial improvement in outcome could be achieved for a/mSTS [5].
Guidelines generally advocate multimodal treatment at specialized centers with a multidisciplinary approach consisting of systemic medical treatment (CTx) and surgery (Sx) and/or radiotherapy (RTx) [6]. Hereby, nomograms are utilized in prediction of treatment outcome in localized disease or counseling towards adjuvant therapies [7, 8]. Also, clinical parameters like tumor localization or histopathological grade have been identified to significantly impact outcome and reflect critical parameters once counseling patients [9, 10]. In particular, once a patient enters the setting of a/mSTS disease stage, the prognosis becomes dismal and risk benefit evaluation of therapeutic aggressiveness is crucial to ensure optimal palliative benefit. However, hardly any valid tool is established for prognostication for therapy aggressiveness in a/mSTS patients.
Sarcopenia, which refers to the depletion of skeletal muscle has emerged as an independent predictor of outcome in a variety of different cancers [11–15]. Further on, it is easily accessible in routine CT-scans, wherein the skeletal muscle index (SMI) is measured for sarcopenia determination. Body composition parameters, like the SMI, can have an impact on risk assessment and clinical decision making in different cancer types. For instance, in advanced gastric cancer, sarcopenia was shown to be an independent prognostic factor for shorter OS and in colorectal cancer sarcopenia was also associated with reduced OS, as well as diminished progression free survival (PFS) [15, 16].
Therefore, body composition parameters are highly interesting to be evaluated in a/mSTS patients, who urgently need fast forward prognostication systems to avoid over- or undertreatment. To our knowledge sarcopenia has not been evaluated as prognostic marker for a/mSTS. Thus, the aim of this study was to analyze the impact of sarcopenia on outcome in a cohort of a/mSTS patients treated at a tertiary center, retrospectively.
Materials and methods
Patient data
181 patients over 18 years with a/mSTS treated at the Hannover Medical School between 12/1998 and 05/2016 were identified retrospectively. Patient data were extracted from the digital charts archive and the local clinical tumor registry. Data analysis was done in an anonymized manner in accordance with the declaration of Helsinki [17]. Patients with soft tissue sarcoma, who received palliative CTx with or without measurable disease were included. All soft tissue sarcoma histiotypes were permitted, except for Gastrointestinal Stroma Cell Tumors (GIST). Availability of an abdominopelvic CT scan of diagnostic quality within 14 days prior to start of CTx was required. CT scans were ineligible if readability was rendered impossible e.g. through metal artefacts or tumor invasion.
Clinical data, tumor- and treatment characteristics including age, sex, height, Eastern Cooperative Oncology Group performance status (ECOG), tumor grading, tumor size and localization, initial resection status and mode of metastatic spread as well as therapeutic lines and agents, additional surgeries and application of radiotherapy were obtained [18]. Mode of metastatic spread describes the occurrence of metastases in respect to initial diagnosis, synchronous spread was defined as diagnosis of metastatic disease within three months of initial diagnosis. Multimodal therapy was defined as at least one tumor related surgery (Sx) and/or tumor related radiotherapy (RTx) in addition to CTx. Predefined primary endpoint is overall survival (OS). Secondary endpoints are progression free survival (PFS), disease control rate (DCR) and overall response rate (ORR) of first palliative CTx, based on routine radiological judgment. Subgroup analysis of sarcopenic and non-sarcopenic patients were predefined.
Assessment of sarcopenia
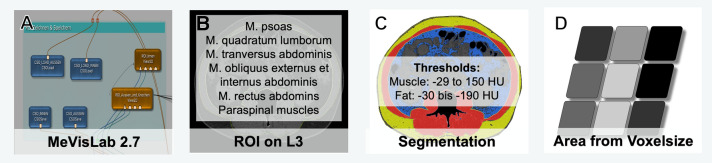
Sarcopenia was measured in pre-treatment CT scans using a specifically self-designed software-tool in MeVisLAB (MeVisLAB 2.7, Fraunhofer MEVIS, Bremen, Germany) for image evaluation by experienced radiology specialists (DS, BH). After importing axial DICOM images of the abdomen with a reconstruction interval of 5 mm and a standard soft reconstruction kernel into the MeVisLAB software, a cross sectional image on the level of mid-L3 vertebra showing both transverse processes was selected. The body compartments were segmented manually with a closed spline region of interest (ROI) in the selected slice. Within these areas, Hounsfield unit (HU) thresholds were defined for skeletal muscle with a range of −29–150 HU, according to consensus-based recommendations [19]. The derived area for muscle was calculated from the number of voxels and voxel size within the ROI (Fig. 1).
Fig. 1.
Workflow measurement setup of the body composition parameters analysis using MeVisLab 2.7
This area constituted the basis for calculating the skeletal muscle index (SMI), normalized by height, according to the formula:
The gender specific cut-off values defining sarcopenia for the SMI were obtained by optimal fitting method from SMI values of the cohort [20]. This resulted in a sarcopenia defining cut-off for men of ≤ 44 and for women of ≤ 38.
Statistical analysis
Data were analyzed with descriptive and inferential statistics. Categorical data were recorded as absolute frequencies, percentages and, if applicable, range. P values are based on different tests as appropriate. Shapiro–Wilk test was utilized to verify Gaussian distribution of the SMI. The cohort was stratified for sarcopenia, based on the appropriate SMI cut-off value, as mentioned above.
OS was defined as time from first palliative CTx until death or last visit. PFS was defined as time from first palliative CTx to progression or death by clinical or radiological judgement. Kaplan Meier analysis with log-ranks were administered for PFS and OS calculation. Univariate and multivariate Cox-proportional hazard regression analysis was employed to evaluate variables for their prognostic value. Variables with a P ≤ 0.2 in univariate analysis were subjected to a multivariate Cox-Regression analysis. Hazard ratios (HRs) and confidence intervals (CIs) were estimated. The alpha level for testing significance was set at P < 0.05. Statistical analysis was performed using SPSS v24 (IBM corp., Armonk, USA).
Results
Patient, tumor and treatment characteristics
89 (49%) of 181 a/mSTS patients were eligible for this retrospective analysis, according to predefined in- and exclusion criteria. The cohort consists of slightly more male than female patients (n = 49, 55.1%) with a median age of 60 (range (r), 20–79) years at diagnosis of a/mSTS. 70 (78.7%) patients showed a good performance status (ECOG < 1). At initial diagnosis 81 (91.0%) sarcomas were classified as deep according to TNM rules and 69 (77.5%) sarcomas had a diameter of > 5 cm [21]. The histopathological grade was ≥ 2 in 74 (83.1%) sarcomas. Leiomyosarcoma and Sarcoma NOS were the most common histologic entities (30.3%, respectively 33.7%) (Table 1).
Table 1.
Characteristics of patients (pts) with soft tissue sarcoma in dependence of sarcopenia
| Variables | All pts | Non-sarcopenic pts | Sarcopenic pts | P |
|---|---|---|---|---|
| n = 89 (100%) | n = 61 (68%) | n = 28 (32%) | ||
| Age at diagnosis STS (years), median (range) | 54 (18–79) | 52 (18–77) | 59 (24–79) | 0.055 |
| Age at diagnosis a/mSTS (years), median (range) | 60 (20–79) | 57 (20–77) | 62 (24–79) | 0.025 |
| Gender | ||||
| Male, n (%) | 49 (55.1) | 35 (57.4) | 14 (50) | 0.516 |
| Female, n (%) | 40 (44.9) | 26 (42.6) | 14 (50) | |
| Tumor site* | ||||
| Superficial, n (%) | 5 (5.6) | 4 (6.6) | 1 (3.6) | 0.850 |
| Deep, n (%) | 81 (91.0) | 55 (90.2) | 26 (92.9) | |
| NE, n (%) | 3 (3.4) | 2 (3.3) | 1 (3.6) | |
| Size* | ||||
| ≤ 5 cm, n (%) | 16 (18) | 10 (16.4) | 6 (21.4) | 0.826 |
| > 5 cm, n (%) | 69 (77.5) | 48 (78.7) | 21 (75) | |
| NE, n (%) | 4 (4.5) | 3 (4.9) | 1 (3.6) | |
| Grading* | ||||
| 1, n (%) | 5 (5.6) | 5 (8.2) | 0 (0) | 0.288 |
| ≥ 2, n (%) | 74 (83.1) | 49 (80.3) | 25 (89.3) | |
| NE, n (%) | 10 (11.2) | 7 (11.5) | 3 (10.7) | |
| Resection status* | ||||
| R0, n (%) | 45 (50.6) | 34 (55.7) | 11 (39.3) | 0.145 |
| R1, n (%) | 9 (10.1) | 7 (11.5) | 2 (7.1) | |
| R2, n (%) | 5 (5.6) | 3 (4.9) | 2 (7.1) | |
| RX, n (%) | 10 (11.2) | 4 (6.5) | 6 (21.4) | |
| NE, n (%) | 20 (22.4) | 13 (21.3) | 7 (25.0) | |
| Metastatic spread | ||||
| Metachronous, n (%) | 54 (60.7) | 36 (59.0) | 18 (64.3) | |
| Synchronous, n (%) | 35 (39.3) | 25 (41.0) | 10 (35.7) | 0.637 |
| ECOG-Status at diagnosis of a/m STS | ||||
| 0, n (%) | 70 (78.7) | 47 (77.0) | 23 (82.1) | 0.586 |
| ≥ 1, n (%) | 19 (21.3) | 14 (23.0) | 5 (17.9) | |
| Metastasis at diagnosis of a/m STS | ||||
| Lung metastases, n (%) | 51 (57.3) | 37 (60.7) | 14 (50.0) | 0.345 |
| Liver metastases, n (%) | 16 (18.0) | 10 (16.4) | 6 (21.4) | 0.566 |
| Lymph node metastases, n (%) | 16 (18.0) | 11 (18.0) | 5 (17.9) | 0.984 |
| Soft tissue metastases, n (%) | 17 (19.1) | 10 (16.4) | 7 (25.0) | 0.337 |
| Cerebral metastases, n (%) | 2 (2.2) | 2 (3.3) | 0 | |
| Bone metastases, n (%) | 14 (15.7) | 9 (14.8) | 5 (17.9) | 0.709 |
| Primary site recurrence, n (%) | 22 (24.7) | 13 (21.3) | 9 (32.1) | 0.271 |
| Other, n (%) | 15 (16.9) | 9 (14.8) | 6 (21.4) | 0.435 |
| Number of organs with metastasis, median, (range) | 1 (1–5) | 2 (0–5) | 1 (0–5) | 0.420 |
| Histologic subtype | 0.499 | |||
| Leiomyosarcoma, n (%) | 27 (30.3) | 17 (27.9) | 10 (35.7) | |
| Liposarcoma, n (%) | 7 (7.9) | 6 (9.8) | 1 (3.6) | |
| Sarcoma NOS, n (%) | 30 (33.7) | 5 (8.2) | 2 (7.1) | |
| Other, n (%) | 25 (28.1) | 19 (31.1) | 6 (21.4) | |
*At time of primary diagnosis
The median period from initial diagnosis to diagnosis of a/mSTS was 7 (r, 0–207) months, with a predominance of metachronous metastatic spread (n = 54, 60.7%), dominated by a pulmonary pattern (n = 51, 57.33%) (Table 1).
All 89 patients received a median of 2 (r, 1–8) lines of CTx. Additionally, 52 (58.4%) patients received a multimodal therapy of CTx and either surgery, radiotherapy or both. Overall, patients received a median of 4 (r, 1–11) oncological interventions (Table 2).
Table 2.
Characteristics of therapy in a/m STS patients (pts) in dependence of sarcopenia
| Variables | All pts | Non-sarcopenic pts | Sarcopenic pts | P |
|---|---|---|---|---|
| n = 89 (100%) | n = 61 (%) | n = 28 (%) | ||
| Number of administered CTx, median (range) | 2 (1–8) | 2 (1–8) | 1.5 (1–7) | 0.112 |
| Number of administered Sx, median (range) | 0 (0–7) | 0 (0–7) | 0 (0–4) | 0.733 |
| Number of administered RTx, median (range) | 0 (0–4) | 0 (0–3) | 0 (0–4) | 0.482 |
| Cumulative no. of therapies, median (range) | 4 (1–11) | 4 (1–11) | 2.5 (1–11) | 0.132 |
| MT – (CTx only), n (%) | 37 (41.6) | 22 (36.1) | 15 (53.6) | 0.122 |
| MT + (CTx + Sx/RTx), n (%) | 52 (58.4) | 39 (63.9) | 13 (46.4) | |
| Medical treatment | ||||
| Doxorubicin, n (%) | 34 (38.2) | 25 (41.0) | 9 (21.4) | |
| Doxorubicin + Ifosfamide, n (%) | 26 (29.2) | 20 (32.8) | 6 (21.4) | |
| Other, n (%) | 29 (32.6) | 16 (26.2) | 13 (46.4) | |
| Clinical response (CTx 1) | 0.089 | |||
| CR, n (%) | 1 (1.1) | 0 | 1 (3.6) | |
| PR, n (%) | 20 (22.5) | 17 (27.9) | 3 (10.7) | |
| SD, n (%) | 16 (18.0) | 13 (21.3) | 3 (10.7) | |
| PD, n (%) | 40 (44.9) | 24 (39.3) | 16 (57.1) | |
| NE, n (%) | 12 (13.5) | 7 (11.5) | 5 (17.9) | |
| ORR, n (%) | 21 (23.6) | 17 (27.9) | 4 (14.3) | 0.161 |
| DCR, n (%) | 37 (41.6) | 30 (49.2) | 7 (25) | 0.032 |
| Treatment discontinued, n (%) | 58 (65.2) | 40 (65.6) | 18 (64.4) | 0.906 |
| Reason for discontinuation of CTx | 0.849 | |||
| Progression n (%) | 42 (75.0) | 29 (76.3) | 13 (72.2) | |
| Toxicity, n (%) | 12 (21.4) | 8 (21.1) | 4 (22.2) | |
| Other, n (%) | 2 (3.6) | 1 (2.6) | 1 (5.6) | |
CTx chemotherapy, Sx surgery, RTx Radiotherapy, Cumulative number of therapies: Sum of administered CTx, Sx and RTx, MT multimodal therapy, CR complete response, SD stable disease, PR partial response, Mixed: mixed response, PD progressive disease, ORR objective response rate (CR + PR), DCR Disease control rate (CR + PR + SD)
Patient characteristics in dependence of sarcopenia
The median SMI was 47.7 (26.7–69.6) in men and 40.2 (30.3–64.9) in women. Overall 28 (32%) patients suffered from sarcopenia at diagnosis of a/mSTS (Table 1). No significant difference for gender, tumor stage, tumor grade and tumor localization, etc. was observed in dependence of sarcopenia (Table 1).
At initial diagnosis sarcopenic patients tended to be older than non-sarcopenic patients (p = 0.055) while the age was significantly different at onset of a/mSTS (P = 0.025). Sarcopenic patients overall tended to receive fewer oncologic interventions than non-sarcopenic patients (median 2.5, r 1–11 vs. 4, r 1–11, P = 0.132), including less lines of CTx (median 1.5, r 1–7 vs. 2, r 1–8, p = 0.112). Also, sarcopenic patients seemed to receive a multimodal treatment approach less often (Table 2).
Efficacy and outcome of a/mSTS patients in dependence of sarcopenia
Response was numerically higher in non-sarcopenic patients with an ORR of 27.9% compared to 14.3% in sarcopenic patients, but did not reach statistical significance (P = 0.161). The DCR of first line CTx was significantly higher in non-sarcopenic patients (49.2% vs. 25%, P = 0.032) (Table 2).
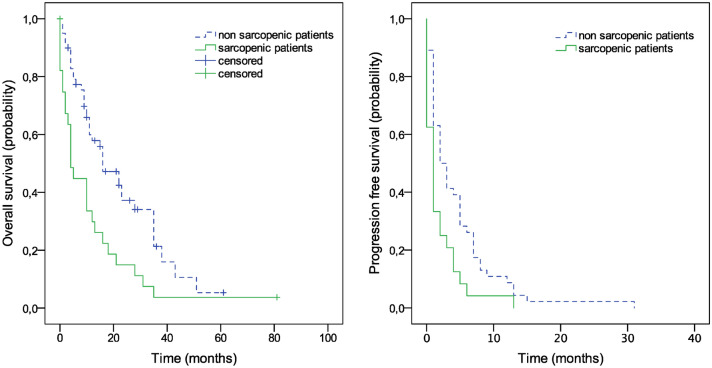
PFS of first line CTx differed significantly between sarcopenic and non-sarcopenic patients (median 2 months (95% CI, 0.67–3.32), vs. 1 month (95%CI, 0.35–1.65), log-rank P = 0.006) (Fig. 2). Median OS of sarcopenic patients was significantly lower compared to non-sarcopenic patients, with 4 months (95% CI, 1.9–6.0) compared to 16 months (95% CI, 8.8–23.2) months in non-sarcopenic patients (log-rank P = 0.002) (Fig. 2).
Fig. 2.
Kaplan–Meier plots. Overall Survival and Progression Free Survival
Sarcopenia as risk predictor for OS and PFS
Univariate analysis for overall survival showed significantly impaired outcome for sarcopenic patients, numbers of involved organ systems by metastasis, as well as a performance status of ECOG ≥ 1 (Table 3). However, subsequent multivariate analysis confirms sarcopenia and ECOG ≥ 1 as independent risk predictors for OS (HR 2.40 [95%-CI, 1.44–4.00], P < 0.001; respectively HR 2.34 [95%-CI, 1.36–4.03], P = 0.002) (Table 3).
Table 3.
Cox regression analysis Overall Survival
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sarcopenia | 2.11 (1.28–3,47) | 0.003 | 2.40 (1.44–4.00) | 0.001 |
| Age at diagnosis a/mSTS | 1.01 (0.99–1.03) | 0.392 | ||
| Gender | 1.63 (0.98–2.73) | 0.061 | 1.66 (.98–2.80) | 0.060 |
| Tumor characteristics, at initial diagnosis | ||||
| Site (superficial vs. deep) | 0.60 (0.27–1.33) | 0.209 | ||
| Size (≤ 5 cm vs. > 5 cm) | 1.02 (0.62–1.68) | 0.925 | ||
| Grade (G1 vs. G2 + 3) | 1.10 (0.64–1.91) | 0.725 | ||
| Resection status (R0 vs. Other) | 1.04 (0.81–1.34) | 0.738 | ||
| Histologic subtype | ||||
| Leiomyosarcoma | 1.00 | 0.470 (df = 3) | ||
| Liposarcoma | 1.42 (0.52–3.87) | 0.492 | ||
| Sarcoma NOS | 1.08 (0.57–2.03) | 0.815 | ||
| Other | 1.59 (0.85–2.97) | 0.150 | ||
| Metastasis at diagnosis a/mSTS (no/yes) | ||||
| Lung metastases | 0.89 (0.55–1.46) | 0.650 | ||
| Liver metastases | 1.16 (0.64–2.11) | 0.631 | ||
| Lymph node metastases | 1.41 (0.71–2.78) | 0.328 | ||
| Soft tissue metastases | 1.07 (0.57–2.02) | 0.833 | ||
| Cerebral metastases | 5.94 (0.75–47.18) | 0.092 | 4.62 (0.55–38.70) | 0.158 |
| Bone metastases | 1.41 (0.73–2.71) | 0.306 | ||
| Primary site recurrence | 1.43 (0.80–2.53) | 0.227 | ||
| Other | 1.81 (0.95–3.46) | 0.073 | 1.07 (0.59–1.93) | 0.836 |
| No. organs with metastasis (1/ > 1) | 1.95 (1.16–3.26) | 0.011 | 1.69 (0.97–2.94) | 0.064 |
| Mode of metastatic spread (synchronous vs. metachronous) | 0.70 (0.43–1.15) | 0.159 | 0.62 (0.37–1.05) | 0.073 |
| MT + (CTx + Sx/RTx), vs. MT – (CTx only) | 0.86 (0.52–1.41) | 0.538 | ||
| ECOG at diagnosis a/mSTS (0/ ≥ 1) | 2.33 (1.23–4.38) | 0.009 | 2.34 (1.36–4.03) | 0.002 |
With regard to PFS, sarcopenia and age at diagnosis of a/mSTS were identified as significantly prognostic in univariate analysis. However, multivariate analysis could not confirm these parameters as independent risk factors for PFS of first line medical treatment (Table 4).
Table 4.
Cox regression analysis progression free survival
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sarcopenia | 1.84 (1.11–3.06) | 0.019 | 1.66 (0.98–2.83) | 0.061 |
| Age at diagnosis a/mSTS | 1.02 (1.00–1.04) | 0.049 | 1.01 (0.99–1.03) | 0.156 |
| Gender | 1.28 (0.80–2.07) | 0.307 | ||
| Tumor characteristics, at initial diagnosis | ||||
| Site (superficial/deep) | 0.78 (0.30–2.04) | 0.611 | ||
| Size (≤ 5 cm/ > 5 cm) | 0.93 (0.53–1.65) | 0.811 | ||
| Grade (G1/G2 + 3) | 1.17 (0.70–1.95) | 0.553 | ||
| Resection status (R0/Other) | 1.34 (0.76–2.39) | 0.315 | ||
| Histologic subtype | ||||
| Leiomyosarcoma | 1 (df = 3) | 0.980 | ||
| Liposarcoma | 0.86 (0.36–2.04) | 0.725 | ||
| Sarcoma NOS | 0.97 (0.53–1.79) | 0.922 | ||
| Other | 0.90 (0.48–1.70) | 0.750 | ||
| Metastasis at diagnosis a/mSTS (no/yes) | ||||
| Lung metastases | 0.87 (0.53–1.42) | 0.565 | ||
| Liver metastases | 1.04 (0.62–1.76) | 0.879 | ||
| Lymph node metastases | 1.31 (0.76–2.28) | 0.331 | ||
| Soft tissue metastases | 1.09 (0.65–1.84) | 0.742 | ||
| Cerebral metastases | 1.88 (0.26–13.77) | 0.534 | ||
| Bone metastases | 1.25 (0.69–2.28) | 0.467 | ||
| Primary site recurrence | 1.28 (.76–2.16) | 0.357 | ||
| Other | 1.05 (.62–1.77) | 0.863 | ||
| No. organs w/metastasis (1/ > 1) | 1.37 (0.85–2.22) | 0.196 | 1.35 | 0.217 |
| Mode of metastatic spread (synchronous/metachronous) | 0.89 (0.55–1.45) | 0.892 | ||
| MT + (CTx + Sx/RTx), vs. MT – (CTx only) | 0.76 (0.47–1.24) | 0.274 | ||
| ECOG at diagnosis a/mSTS (0 / ≥ 1) | 1.11 (0.68–1.81) | 0.679 | ||
Discussion
Patients with a/mSTS do have a dismal prognosis, which renders patient counseling a challenge. Up to our knowledge, we report for the first time on the impact of radiologically defined sarcopenia in a cohort of a/mSTS patients prior to palliative medical therapy, which showed a significant association with the outcome parameter OS. Sarcopenia impacted also other efficacy parameter, but independence of sarcopenia as a predictor could not be shown.
89 of 181 a/mSTS patients who received palliative treatment were eligible for this retrospective analysis. This relatively small number is in part owed to the low incidence of soft tissue sarcoma, as well as to the pre-defined inclusion criteria [22]. In particular, the mandated CT-scan within a timeframe of 14 days prior to CTx initiation limited the number of eligible patients. Although our reported cohort of eligible patients is small, it resembles typical characteristics of other STS cohorts, e.g. like previously reported in 78.527 STS patients of the SEER register [23]. Similarities include the important confounder of histology STS subtype distribution, with the most common being sarcoma NOS, leiomyosarcoma and liposarcoma as well as grade ≥ 2 in the majority of patients [23]. Also, age, an important confounder, as well as gender distribution is comparable in both cohorts with a median age of 54 years at primary diagnosis of STS, 58 years at diagnosis of a/mSTS, respectively. In terms of outcome, the median OS of 12 months of the overall cohort is comparable to other recent studies on a/mSTS with similar CTx regimes [24, 25]. The PFS of the overall cohort with a median of 2 months (95%CI 1.40–2.60) in this cohort is lower in comparison to clinical trial results, which is possibly confounded by the real-world population, as illustrated by the performance status, as well as by the selection process for the current study [25]. None the less, all mentioned parameters suggest, to a certain extent, that the analyzed cohort reflects a representative a/mSTS real-world cohort. Although the cohort was quite small, sarcopenia maintained significance as an independent prognostic risk factor, which underlines the strength of this biological stratum.
Stratified for sarcopenia, comparison of the subgroups showed that tumor characteristics between sarcopenic and non-sarcopenic patients mostly do not differ significantly (Table 1). At diagnosis of a/mSTS sarcopenic patients are significantly older (P = 0.025). This coincides with other studies. Sarcopenia is highly prevalent in cohorts with solid tumors, reported incidences here vary greatly with ranges from 11 to 74% [26]. It needs to be considered, though, that sarcopenia is also highly prevalent in elderly cancer-free cohorts with percentages ranging from 5 to 50% [27]. Consistent with this, sarcopenic patients show a statistic trend towards being older at initial diagnosis (P = 0.055) (Table 1).
With regard to therapy, sarcopenic patients tend to receive less aggressive multimodal therapies than non-sarcopenic patients. Furthermore, there is lower usage of doxorubicin + ifosfamide in sarcopenic patients (41.0 vs. 21.4%), indicating that a less aggressive therapy is more frequently chosen. This supports the assumption that there is already a selection towards less intensive treatment in sarcopenic patients based on clinical judgement. Notwithstanding, the treated sarcopenic patients still tend to profit less from first-line CTx with best clinical response being tumor progression in 57% of cases compared to 39% in non-sarcopenic patients, although the discontinuation rate due to toxicity seemed considerably equal between subgroups. Consequentially, the DCR is significantly lower in sarcopenic patients (P = 0.032). Studies on other solid tumors have found an association between sarcopenia and toxicity, and there is another body composition marker, namely the body mass index (BMI), that has been identified as a risk factor for toxicity in a/mSTS [28, 29]. However, this correlation could not be found in our cohort (Data not shown).
Multivariate analysis identified ECOG ≥ 1 and sarcopenia as independent predictors for impaired overall survival. Whether or not, sarcopenia and the performance status describe the same biological phenomenon, or need to be addressed as synergistic parameters was not evaluated by our analysis. However, other studies have found age, gender, histiotype and grade to be prognostic in a/mSTS [9, 10]. With regard to grade, we selected chemo-sensitive STS for medical treatment. This led to inclusion of only 5 patients (5.6%) with grade 1 STS, which renders our study underpowered to perform such a comparison. The finding of ECOG performance status being prognostic is consistent with previous reports in STS [30]. Female gender shows a trend (P = 0.060) to have a favorable prognosis, a correlation which is also found in a retrospective review of a large SEER cohort with STS [31].
Most importantly, the CT-derived marker sarcopenia results in a significant difference in OS of 4 months for sarcopenic compared to 16 months of non-sarcopenic patients [HR 2.4 (95-%CI: 1.44–4), P = 0.002]. A correlation between sarcopenia and prognosis has already been found in a multitude of different cancer entities, but studies showing this also being applicable to STS are scarce, especially in a setting of a/mSTS. In a study investigating body-composition in STS, sarcopenia did not negatively affect OS [32]. Comparability is limited though, because different to our study, that study investigated localized as well as metastatic disease. In two other analyses with higher proportions of patients with more advanced disease, association of sarcopenia with OS was found [33, 34]. Therefore, the impact of sarcopenia seems to be more significant in a/mSTS compared to localized disease stages and this might be attributed to higher levels of inflammation found in advanced and metastatic disease linked to cancer cachexia [35]. This might contribute to the significant impact of sarcopenia on OS in our cohort of exclusively a/mSTS patients.
Also, we found a significant difference in PFS, albeit small in absolute numbers with 1 month in sarcopenic patients compared 2 months in the non-sarcopenic group. Reflecting the separation potency of sarcopenia, as well as its increasing accessibility, prediction of a/mSTS through SMI seems to be highly interesting in treatment counseling in a/mSTS. However, sarcopenia could not be identified as an independent marker for PFS in our analysis, underscoring its relevance as a prognostic marker.
Although the general definition of sarcopenia as muscle depletion from any cause is widely accepted, as is its principle method of measuring via the CT-derived marker SMI, the variation in reported incidences may partly be explained by the usage of different sarcopenia-defining cut-off values and different methods of measurement in detail [37, 38].
In this study, sarcopenia-defining cut-off values were found by optimal fitting method, the optimal cut-off here being defined as the point with the most significant log-rank test split [20]. The herewith obtained values are close to 2 standard deviations (SD) below the median SMI of a healthy cohort, a method traditionally chosen to find pathology defining cut-off values [39, 40]. There are proposed consensus cut-off values for the SMI available in the literature. However, these consensus cut-off values show a different deviation for men and women compared to a healthy cohort with no apparent explanation [41, 42]. This made the approach of defining cut-off values by optimal fitting method seem more viable and this is also widely practiced [12]. Another possible source of deviation in reported SMI values may be attributed to the use of different software solutions. In this study a self-designed, purpose-built albeit unvalidated software within the MeVisLAB framework is utilized, whereas other studies use commercially available software solutions [33, 34].
Also, this analysis is obviously limited due to its sample size and selection of patients. However, the cohort’s characteristics with regard to demographics and disease resemble those of larger chemo-sensitive STS cohorts.
A limiting factor in using sarcopenia as a marker in routine care is the current technique of measuring. Although CT-scans are routinely performed for staging purposes of STS and thus would be available for body composition analysis, their time-consuming calculation would overly stretch resources of radiology departments in daily care. With the advent of artificial intelligence algorithms, though, it is likely that parameters like the SMI will be among the first that could become readily available as a “byproduct” in routine CT-reports [43].
Further on, comorbidities were not assessed in our analyses, as they were considered negligible in patients with a/mSTS due to their dismal prognosis [36]. However, we cannot rule out a biasing effect of comorbidities in our analyses.
Ultimately, the current finding, that sarcopenia is independently associated with OS, SMI might reflect a powerful tool in prognostication and to some extent in treatment counseling of a/mSTS patients, in terms of choosing aggressive or less aggressive palliative therapy approaches. At length, only prospective trials or lager cohorts will generate higher evidence in relation to this hypothesis.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received for conducting this study.
Declarations
Conflict of interest
All authors have no conflicts of interest to declare that are relevant to the content of this article.
Human and animal rights
All datasets used for this study are permanently stored at the institutions’ servers and are available from the corresponding author on reasonable request. All human data are stored anonymously.
Ethics approval
Ethics approval was waived by the local ethics committee of the Hannover Medical School (Registration number 3658-2017). The ethical recommendations of the Declaration of Helsinki were adhered to All authors have approved the final manuscript for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dennis Strassmann and Bennet Hensen contributed equally.
Contributor Information
Dennis Strassmann, Email: dennis.strassmann@krh.eu.
Bennet Hensen, Email: hensen.bennet@mh-hannover.de.
Philipp Ivanyi, Email: ivanyi.philipp@mh-hannover.de.
References
- 1.Hall F, Villalobos V, Wilky B, et al. Future directions in soft tissue sarcoma treatment. Curr Probl Cancer. 2019;43:300–307. doi: 10.1016/j.currproblcancer.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 2.In GK, Hu JS, Tseng WW, et al. Treatment of advanced, metastatic soft tissue sarcoma: latest evidence and clinical considerations. Ther Adv Med Oncol. 2017;9:533–550. doi: 10.1177/1758834017712963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younger E, Litière S, Le Cesne A, et al. Outcomes of elderly patients with advanced soft tissue sarcoma treated with first-line chemotherapy: a pooled analysis of 12 EORTC Soft tissue and bone sarcoma group trials. Oncologist. 2018;23:1250–1259. doi: 10.1634/theoncologist.2017-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer of soft tissue including heart - Cancer Stat Facts. SEER n.d. https://seer.cancer.gov/statfacts/html/soft.html (Accessed 18 Jun 2020).
- 5.Tap WD, Wagner AJ, Papai Z, et al. ANNOUNCE: a randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox) + olaratumab versus dox + PBO in patients (pts) with advanced soft tissue sarcomas (STS) J Clin Oncol. 2019;37:LBA3–LAB3. doi: 10.1200/JCO.2019.37.18_suppl.LBA3. [DOI] [Google Scholar]
- 6.Casali PG, Abecassis N, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO–EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv51–67. doi: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 7.Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17:671–680. doi: 10.1016/S1470-2045(16)00010-3. [DOI] [PubMed] [Google Scholar]
- 8.Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31:1649–1655. doi: 10.1200/JCO.2012.44.3747. [DOI] [PubMed] [Google Scholar]
- 9.Brennan MF, Antonescu CR, Moraco N, et al. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260:416–422. doi: 10.1097/SLA.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verschoor AJ, Litière S, Marréaud S, et al. Prognostic relevance of distant metastases versus locally advanced disease in soft tissue sarcomas: an EORTC-STBSG database study. Eur J Cancer Oxf Engl. 1990;2018(94):187–198. doi: 10.1016/j.ejca.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Pamoukdjian F, Bouillet T, Lévy V, et al. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr. 2018;37:1101–1113. doi: 10.1016/j.clnu.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 13.Jung AR, Roh J-L, Kim JS, et al. Prognostic value of body composition on recurrence and survival of advanced-stage head and neck cancer. Eur J Cancer. 2019;116:98–106. doi: 10.1016/j.ejca.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Rier HN, Jager A, Sleijfer S, et al. The prevalence and prognostic value of low muscle mass in cancer patients: a review of the literature. Oncologist. 2016;21:1396–1409. doi: 10.1634/theoncologist.2016-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng C-Y, Lin Y-C, Wu JS, et al. Progressive sarcopenia in patients with colorectal cancer predicts survival. Am J Roentgenol. 2018;210:526–532. doi: 10.2214/AJR.17.18020. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Kim YS, Kim EY, et al. Prognostic significance of CT-determined sarcopenia in patients with advanced gastric cancer. PLoS ONE. 2018;13:e0202700. doi: 10.1371/journal.pone.0202700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 18.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Heymsfield BS, Wang Z, Baumgartner RN, et al. Human body composition: advances in models and methods. Annu Rev Nutr. 1997;17:527–558. doi: 10.1146/annurev.nutr.17.1.527. [DOI] [PubMed] [Google Scholar]
- 20.Budczies J, Klauschen F, Sinn BV, et al. Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS ONE. 2012;7:e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittekind C, International Union against Cancer, editors (2005) TNM-Klassifikation maligner Tumoren. 6. Aufl., korrigierter Nachdr. Springer Medizin, Heidelberg
- 22.Stiller CA, Trama A, Serraino D, et al. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer. 2013;49:684–695. doi: 10.1016/j.ejca.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Gage MM, Nagarajan N, Ruck JM, et al. Sarcomas in the united states recent trends and a call for improved staging. Oncotarget. 2019;10:2462–2474. doi: 10.18632/oncotarget.26809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 25.Meyer M, Seetharam M. First-line therapy for metastatic soft tissue sarcoma. Curr Treat Options Oncol. 2019 doi: 10.1007/s11864-019-0606-9. [DOI] [PubMed] [Google Scholar]
- 26.Shachar SS, Williams GR, Muss HB, et al. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Morishita S. Prevalence of sarcopenia in cancer patients: review and future directions. Int J Phys Med Rehabil. 2016 doi: 10.4172/2329-9096.1000342. [DOI] [Google Scholar]
- 28.Barret M, Antoun S, Dalban C, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–589. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 29.Vincenzi B, Badalamenti G, Armento G, et al. Body mass index as a risk factor for toxicities in patients with advanced soft-tissue sarcoma treated with trabectedin. Oncology. 2018;95:1–7. doi: 10.1159/000487266. [DOI] [PubMed] [Google Scholar]
- 30.Penel N, Glabbeke MV, Mathoulin-Pelissier S, et al. Performance status is the most powerful risk factor for early death among patients with advanced soft tissue sarcoma: the European Organisation for Research and Treatment of Cancer – Soft Tissue and Bone Sarcoma Group (STBSG) and French Sarcoma Group (FSG) study. Br J Cancer. 2011;104:1544–1550. doi: 10.1038/bjc.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, Xu G, Wu H, et al. The nomogram for early death in patients with bone and soft tissue tumors. J Cancer. 2020;11:5359–5370. doi: 10.7150/jca.46152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RJ, Alamanda VK, Hartley KG, et al. Sarcopenia does not affect survival or outcomes in soft-tissue sarcoma. Sarcoma. 2015 doi: 10.1155/2015/146481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boutin RD, Katz JR, Chaudhari AJ, et al. Association of adipose tissue and skeletal muscle metrics with overall survival and postoperative complications in soft tissue sarcoma patients: an opportunistic study using computed tomography. Quant Imaging Med Surg. 2020;10:1580–1589. doi: 10.21037/qims.2020.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phan EN, Thorpe SW, Wong FS, et al. Opportunistic muscle measurements on staging chest CT for extremity and truncal soft tissue sarcoma are associated with survival. J Surg Oncol. 2020 doi: 10.1002/jso.26077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colloca G, Di Capua B, Bellieni A, et al. Muscoloskeletal aging, sarcopenia and cancer. J Geriatr Oncol. 2019;10:504–509. doi: 10.1016/j.jgo.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Raedkjaer M, Maretty-Kongstad K, Baad-Hansen T, et al. The impact of comorbidity on mortality in Danish sarcoma patients from 2000–2013: a nationwide population-based multicentre study. PLoS ONE. 2018;13:e0198933. doi: 10.1371/journal.pone.0198933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boutin RD, Yao L, Canter RJ, et al. Sarcopenia: current concepts and imaging implications. Am J Roentgenol. 2015;205:W255–W266. doi: 10.2214/AJR.15.14635. [DOI] [PubMed] [Google Scholar]
- 38.Mourtzakis M, Prado CMM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 39.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis report of the European Working Group on Sarcopenia in Older. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 42.Van Der Werf A, Langius JAE, De Van Der Schueren MAE, et al. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr. 2017 doi: 10.1038/s41430-017-0034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burns JE, Yao J, Chalhoub D, et al. A Machine learning algorithm to estimate sarcopenia on abdominal CT. Acad Radiol. 2020;27:311–320. doi: 10.1016/j.acra.2019.03.011. [DOI] [PubMed] [Google Scholar]