Figure 4.
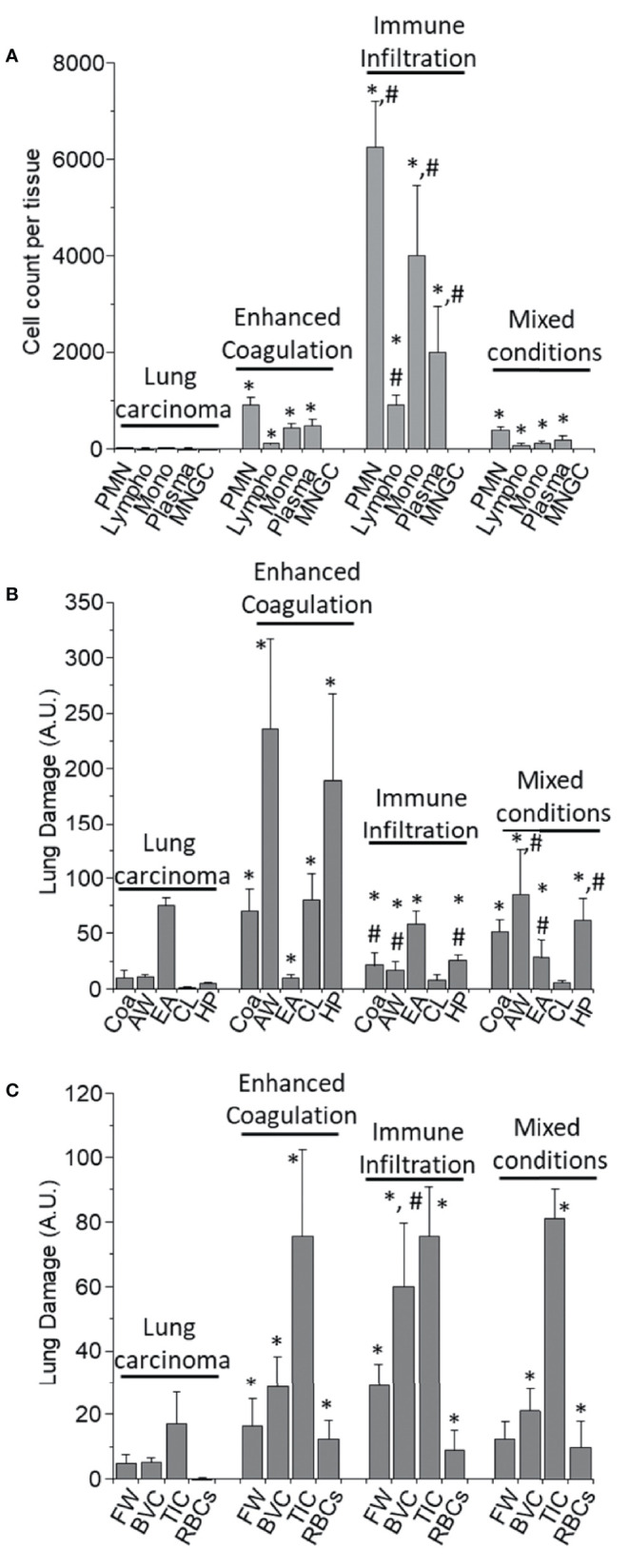
Quantification of the immune infiltration and lung damage. (A) H&E tissue slices were used to quantify the numbers and types of immune cells within the different tissues analyzed. We quantified Polymorphonuclears (PMN), Lymphocytes (Lympho), Monocytes (Mono), Plasma cells (Plasma), and multinucleated giant cells (MNGC) in the different COVID-19 pathology phenotypes, enhanced coagulation, immune infiltration, and mixed conditions, as well as in lung carcinoma samples. In lung carcinoma samples, minimal infiltration of PMN, Lympho, Mono, and Plasma was observed. No MNGC was observed in any of the tissues analyzed. In the enhanced coagulation phenotype, PMN infiltration was the prominent cell type infiltrated. Monocytes, Plasma cells, and lymphocytes migration were minimal compared to lung carcinoma (*p ≤ 0.005 compared to lung carcinoma, n = 4 different individuals with 5 sections each). In the Immune infiltration phenotype, a significantly higher lung infiltration was detected compared to lung carcinoma (*p ≤ 0.0001 compared to lung carcinoma, n = 4 different individuals with 5 sections each) and enhanced coagulation (#p ≤ 0.007 compared to lung carcinoma, n = 4 different individuals with 5 section each). A similar proportion of cells infiltrate the lung, but also with a minimal lymphocyte infiltration. In the mixed conditions, a similar infiltration than in enhanced coagulation was observed (*p ≤ 0.005 compared to lung carcinoma, n = 3 different individuals with five sections each). No MNGC was observed under any of the conditions examined. (B) Quantification of lung damage by examining hemorrhagic/coagulation related lesions (Coa, data expressed in percentage), Alveolar Wall thickness (AW, data expressed as µm), the empty area of the lung (EA, expressed as a percentage), Cell Loss (CL, expressed in percentage and focus mainly in pneumocyte loss into the alveolar space), and hyperplastic Pneumocyte (HP, data expressed as µm). In lung carcinoma, all the aspects examined were similar to the data from multiple publications in healthy conditions. In the enhanced coagulation phenotype (type I), coagulation, alveolar wall, cell loss, and hyperplastic pneumocyte were significantly higher than all the conditions examined. However, a dramatic reduction in the lung’s empty area, a condition required for efficient gas exchange, was detected (*p ≤ 0.00126 compared to lung carcinoma, n = 3 different individuals with five sections each). In the immune infiltration phenotype, significant damage was observed (*p ≤ 0.005 compared to lung carcinoma and #p ≤ 0.007 compared to enhanced coagulation, n = 4 different individuals with 5 sections each). A similar profile than immune infiltration was observed in the mixed conditions with a significant compromise in the EA (#p ≤ 0.00125 compared to enhanced coagulation, n = 4 different individuals with five sections each). (C) Quantification of the lung damage by determining the area with fibrin webs (FW), Blood vessel collagen (BVC), tissue-associated collagen (TIC), and spiky red blood cells (RBCs) using the H&E and trichrome staining was performed. In lung carcinoma, most data were similar to healthy conditions. In all COVID-19 phenotypes, lung damage was elevated equally, except BVC in the immune infiltration higher than in the enhanced coagulation phenotype (*p ≤ 0.005 compared to lung carcinoma and #p ≤ 0.0002 compared to enhanced coagulation phenotype, n = 13 different individuals with 5 section each).
