FIGURE 2.
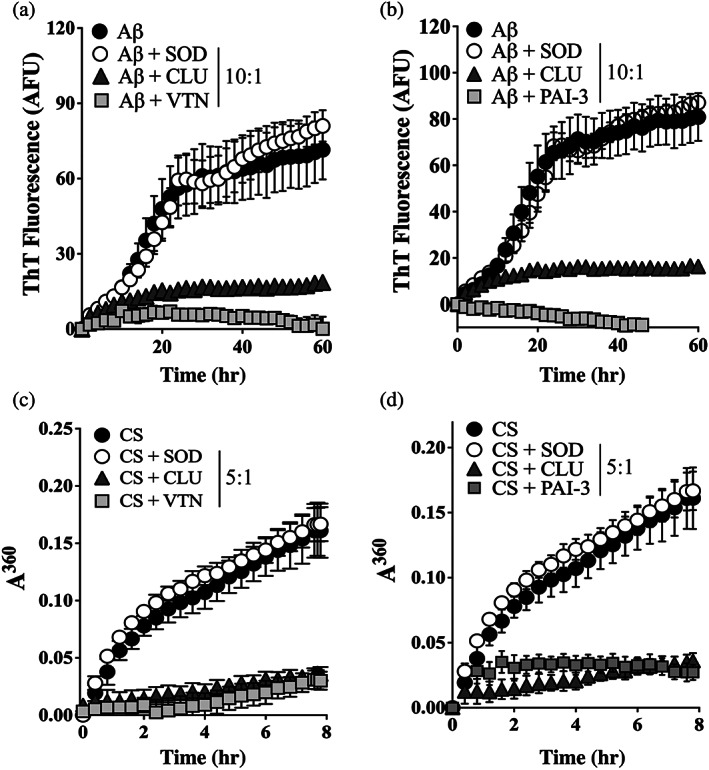
VTN and PAI‐3 inhibit the in vitro aggregation of Aβ1–42 and CS. (a & b) Aβ1–42 (10 μM), supplemented with ThioT (20 μM), was incubated for 60 hr in the presence or absence of 1 μM of (a) VTN or (b) PAI‐3, or superoxide dismutase‐1 (SOD, as non‐chaperone control protein), or clusterin (CLU, a chaperone control protein). Amyloid formation was monitored by measuring ThioT fluorescence. Mean ThioT fluorescence (in arbitrary fluorescence units, AFU) ± SEM (n = 3) are plotted. (c & d) CS (5 μM) was incubated at 43°C for 8 hr in the presence of absence of 1 μM SOD, CLU or (c) VTN, or (d) PAI‐3. Amorphous protein aggregation was monitored as increasing turbidity measured as absorbance at 360 nm (A360). Mean absorbance values ± SEM (n = 3) are plotted. In (a–d), the molar ratios of Aβ1–42 or CS to SOD/CLU/VTN/PAI‐3 are indicated in the respective keys, and in some cases, the error bars are too small to be visible. All results are representative of three independent experiments
