FIGURE 3.
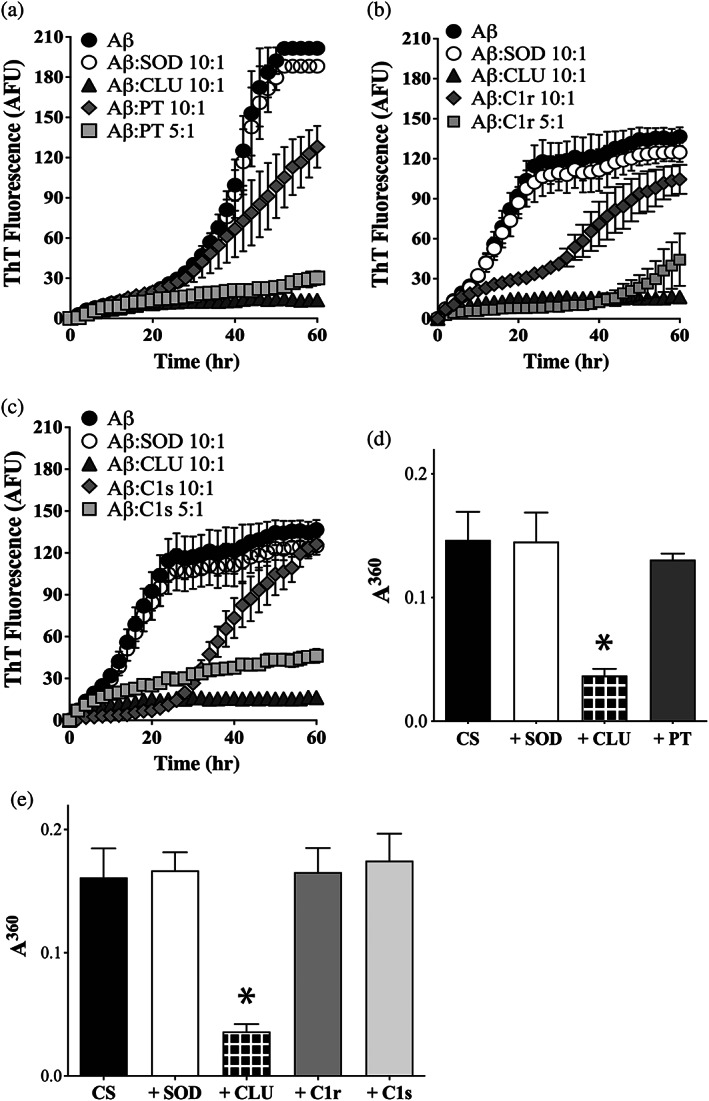
PT, C1r and C1s inhibit the aggregation of Aβ1–42 to form amyloid, but not the amorphous aggregation of CS. (a–c) Aβ1–42 (10 μM) was supplemented with ThioT (20 μM) and incubated for 60 hr in the absence or presence of 1 μM SOD (a non‐chaperone control protein) or CLU (a chaperone control protein). Aggregation reactions were also supplemented or not with either 1 μM or 2 μM of (a) PT, (b) C1r, or (c) C1s. In all cases, the molar ratio of Aβ1–42:test protein is indicated in the respective keys. Amyloid formation was monitored by measuring ThioT fluorescence. Mean ThioT fluorescence values (in arbitrary fluorescence units, AFU) ± SEM (n = 3) are plotted In (a–c), in some cases, the error bars are too small to be visible. (d, e) Endpoint absorbance values for experiments in which 5 μM CS was incubated for 8 hr in the presence or absence of 1 μM of SOD or CLU, or 5 μM of (d) PT or (e) C1r or C1s. Amorphous protein aggregation was monitored as turbidity measured as absorbance at 360 nm (A360). Mean endpoint absorbance values (i.e., at 8 hr) ± SEM (n = 3) are plotted. Each result is representative of two independent experiments. In (d) and (e), the asterisks indicate significant differences in A360 between the CS alone and +CLU treatments (Students t‐test, p < .01); other differences are not significant
